- Search
| Ann Coloproctol > Volume 38(3); 2022 > Article |
|
Abstract
Purpose
Anastomotic bleeding after colorectal surgery is a rare, mostly self-limiting, postoperative complication that could lead to a life-threatening condition. Therefore, prompt management is required. This study aimed to evaluate the efficacy and safety of endoscopic clipping for acute anastomotic bleeding after colorectal surgery.
Methods
We retrospectively reviewed the data of patients pathologically diagnosed with colorectal cancer at National Cancer Center, Korea from January 2018 to November 2020, which presented with anastomotic bleeding within the first postoperative week and were endoscopically managed with clips.
Results
Nine patients had anastomotic bleeding, underwent endoscopic management, and, therefore, were included in this study. All patients underwent laparoscopic (low/ultralow) anterior resection with mechanical double-stapled anastomosis. Anastomotic bleeding was successfully managed through a colonoscopy with clips on the first trial in all patients. Hypovolemic shock occurred in one patient, following anastomotic breakdown.
Anastomotic bleeding after colorectal surgery is a rare, mostly self-limiting, postoperative complication. However, some patients might develop a life-threatening condition. Therefore, prompt and appropriate management is required [1]. Colonoscopy could be an easily accessible and effective diagnostic and therapeutic tool [2]. However, the endoscopic approach with air insufflation during the early postoperative period could negatively affect the anastomosis healing process [3]. Nevertheless, a previous study suggested that anastomotic perforations or breakdown rarely resulted from endoscopy performed Ōēż 6 weeks following gastrointestinal anastomoses [4]. There are several endoscopic treatment options, including injection therapy, argon plasma coagulation (APC), and clipping. Clipping is a mechanical method that could be applied repeatedly.
Thus, we retrospectively reviewed acute anastomotic bleeding cases after colorectal surgery that underwent endoscopic management to evaluate the efficacy and safety of endoscopic clipping for hemostasis during the early postoperative period.
The records of patients pathologically diagnosed with colorectal cancer at National Cancer Center in Goyang, Korea from January 2018 to November 2020 were retrospectively reviewed. Patients with anastomotic bleeding within the first week after colorectal surgery who underwent an endoscopic hemostatic procedure were included in this study. We excluded patients with no evidence of active bleeding during colonoscopic observation. All included patients received a standardized operation regimen and perioperative medication following the hospitalŌĆÖs protocol. Anastomotic bleeding was suspected when hematochezia was detected. There was no absolute criterion for trying further treatments other than conservative treatments or transfusion. However, we considered colonoscopy when massive hematochezia or a significant fall in hemoglobin levels was observed. Bowel preparation was not routinely required for hemostatic colonoscopy. We used a single channel colonoscope (CF-HQ260AL; Olympus, Tokyo, Japan), EZ Clip (Olympus), and Hilzo Clip (BCM, Goyang, Korea). Colonoscopy was performed by gastrointestinal endoscopists. Fig. 1 shows images of the endoscopic clipping procedure in cases 7 and 8 (Table 1). In both patients, the lumen was irrigated with water to improve visualization of the anastomotic integrity. When an active bleeding point was detected, it was clipped. The endoscopic examination ended after hemostasis was achieved.
The following data were retrospectively retrieved from clinicopathological charts: sex, age, body mass index (BMI), comorbidities, history of previous abdominal surgery, tumor location (distance from the anal verge), history of neoadjuvant therapy, operative note, resection extent, method of anastomosis (hand-sewing or stapled), diversion, pathology, time to postoperative hemorrhage, a need for transfusion, hemodynamic instability, post-bleeding endoscopic evaluation, secondary intervention, and anastomosis leakage. We conducted this study in compliance with the principles of the Declaration of Helsinki. The Institutional Review Board of National Cancer Center approved this study (No. NCC2021-0063). The requirement for informed consent was waived because of the retrospective nature of the study.
A total of 1,183 patients underwent surgery for colorectal cancer at National Cancer Center, Korea between January 2018 and November 2020. Among them, 9 patients (0.8%) experienced acute anastomotic bleeding that required endoscopic intervention.
Table 1 presents the patientsŌĆÖ characteristics. Six patients were male, and 3 were female. The mean age was 64.5 years, and the mean BMI was 22.34 kg/m2. One patient had a history of stroke 3 years earlier and was under anticoagulation therapy. Two patients had a history of abdominal surgery. All 9 patients had left-side colon or rectal cancer and underwent laparoscopic (low/ultralow) anterior resection with mechanical double-stapled anastomosis. Three patients had a temporary ileostomy.
Table 2 shows the treatment details. Postoperative hemorrhage occurred at an average of 1.2 days after surgery. Hypovolemic shock occurred in 1 patient. Three patients received red blood cell (RBC) transfusions. No vasoactive drug was injected into any of the patients. All 9 patients were managed successfully with endoscopic clips on the first attempt, and no further intervention was required.
In case 2, hematochezia occurred on postoperative day 1 (hemoglobin level, 5.9 g/dL), leading to hypovolemic shock. The patient was conservatively managed with 5 packs of RBC and stabilized. Thereafter, colonoscopy was performed and anastomotic bleeding was detected, which was successfully controlled with endoscopic clips. Anastomotic leakage was detected on postoperative day 6.
We reviewed anastomotic bleeding cases that occurred within the first week after colorectal cancer surgery and underwent endoscopic management. Hemostasis was achieved in all cases through colonoscopy and clips application. However, anastomotic leakage occurred in 1 patient with hypovolemic shock.
Previous studies reported a risk of anastomotic bleeding in up to 6% of the patients after colorectal anastomosis [3] and a risk of 0.3% within 7 days of surgery [1]. Furthermore, approximately 1% of cases present with massive bleeding and hemodynamic instability [5]. Endoscopy is a minimally invasive diagnostic and therapeutic method that facilitates complete visualization of the anastomotic integrity and provides techniques to treat anastomotic complications. Moreover, it does not require general anesthesia, conferring minimal physiological stress, especially to patients with comorbidities. The patients in this study were endoscopically treated successfully without aggravating their general condition, regardless of their age, comorbidities, or the presence of anticoagulation therapy.
Theoretically, air insufflation during endoscopy could delay the anastomotic healing process; however, endoscopy safety was demonstrated in patients with gastrointestinal [4] and colorectal [6] anastomoses during the early postoperative period. In this study, anastomotic leakage occurred in 1 of the 9 patients. Colorectal anastomotic dehiscence has a multifactorial etiology. Diabetes mellitus might be a relevant etiological factor to the patient in our study [7, 8]. Blood perfusion to the anastomosis and the anastomotic tension are believed to be the most important factors for proper healing. Factors affecting these in our patient, including the duration of surgery, hypovolemic shock, and high ligation of the inferior mesenteric artery, could have been relevant [7, 9, 10]; this patient underwent a right hemihepatectomy for liver metastasis at the time of colorectal anastomosis, with a total operation time of 8 hours.
When luminal bleeding occurs, endoscopic treatment options include injection therapy, thermal coagulation, and clipping [11]. Epinephrine (at a ratio of 1:10,000 or 1:20,000) is the most used injection agent; however, it results in vasoconstriction and enhances platelet aggregation, which might have a negative impact on the anastomotic healing process [6, 11]. APC allows noncontact endoscopic thermal coagulation by delivering a high-frequency electrical current through emitted argon gas; however, its cost is higher than the other modalities, and its use for larger vessels is still controversial [11]. Besides, severe complications of APC, including fistulation, stricture formation, and ischemia, were reported [12].
Nonoperative treatment for postoperative hemorrhage also includes angiographic intervention, and vasopressin infusion through angiographic catheter or embolization. However, both procedures might precipitate bowel ischemia and infarction [13, 14]. Thus, in terms of possible complications, endoscopic clipping could be applied more safely than other endoscopic or angiographic modalities; it is a mechanical method, so it could be applied repeatedly with less damage to perianastomotic vessels than the chemical and electrical methods.
Although all patients in this study were managed successfully through a colonoscopy with clipping on the first trial, Malik et al. [15] suggested that when the first trial fails, repeating it or trying other kinds of endoscopic therapies might be worthwhile in terms of physiologic stress, easy accessibility, and cost-effectiveness before considering reoperation. However, if the luminal view shows severe bleeding, prompt surgical management should be considered.
In conclusion, endoscopic clipping seems to be an effective and safe treatment for hemostasis in acute anastomotic bleeding after colorectal cancer surgery.
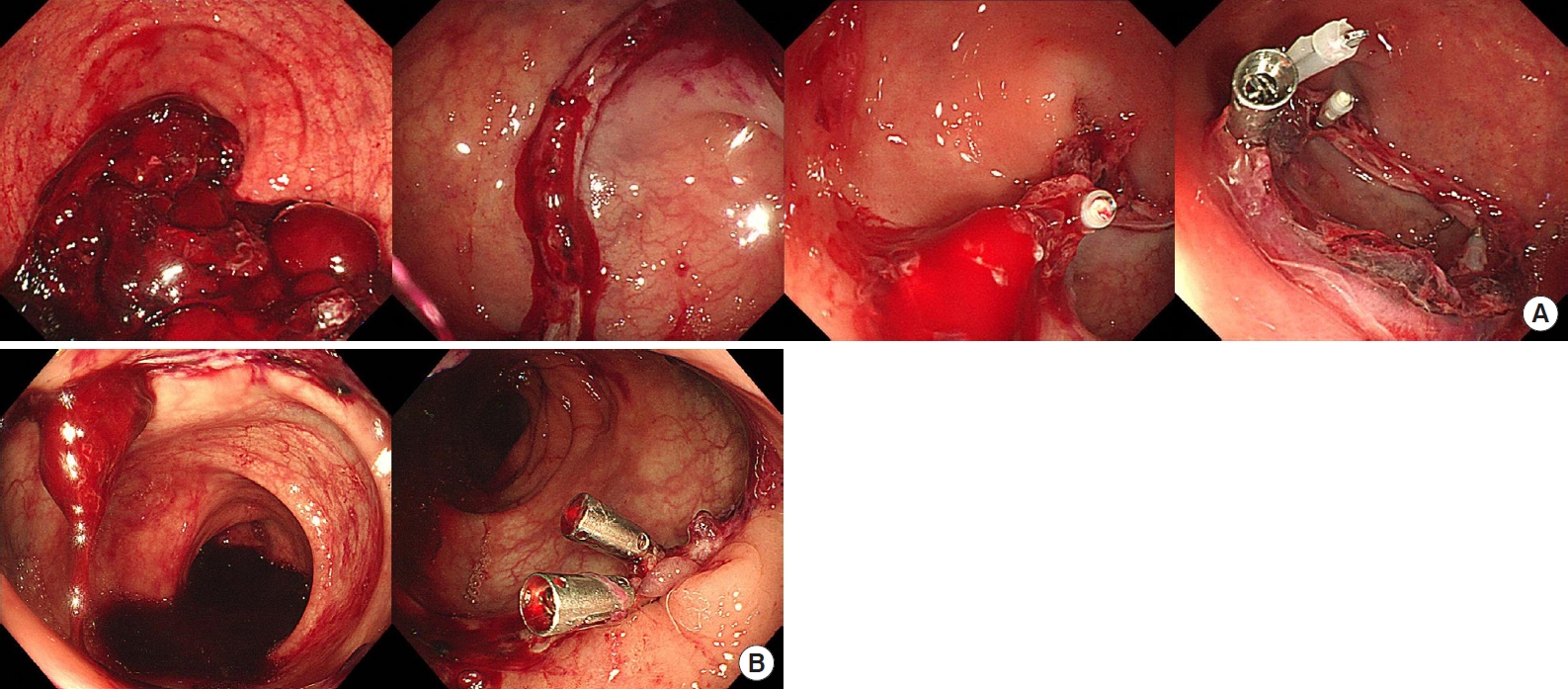
Fig.┬Ā1.
Endoscopic evaluation and hemostasis with hemoclips for anastomotic bleeding. (A) Case 7, 4 clips were applied. (B) Case 8, 2 clips were applied.
Table┬Ā1.
PatientsŌĆÖ characteristics
Table┬Ā2.
Treatment details
REFERENCES
1. Lou Z, Zhang W, Yu E, Meng R, Fu C. Colonoscopy is the first choice for early postoperative rectal anastomotic bleeding. World J Surg Oncol 2014;12:376.



2. ASGE Standards of Practice Committee; Pasha SF, Shergill A, Acosta RD, Chandrasekhara V, Chathadi KV, Early D, et al. The role of endoscopy in the patient with lower GI bleeding. Gastrointest Endosc 2014;79:875ŌĆō85.


3. Besson R, Christidis C, Denet C, Bruyns L, Levard H, Gayet B, et al. Management of postoperative bleeding after laparoscopic left colectomy. Int J Colorectal Dis 2016;31:1431ŌĆō6.


4. Amr MA, Alzghari MJ, Polites SF, Khasawneh MA, Morris DS, Baron TH, et al. Endoscopy in the early postoperative setting after primary gastrointestinal anastomosis. J Gastrointest Surg 2014;18:1911ŌĆō6.


5. Cirocco WC, Golub RW. Endoscopic treatment of postoperative hemorrhage from a stapled colorectal anastomosis. Am Surg 1995;61:460ŌĆō3.

6. Liu W, Lin D, Zhong Q, Su M, Li J, Guo X, et al. Endoscopic management of postoperative anastomotic bleeding in patients with colorectal cancer. Int J Colorectal Dis 2020;35:1703ŌĆō9.


7. McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg 2015;102:462ŌĆō79.


8. Ben─Źurik V, ┼Ākrovina M, Mart├Łnek L, Barto┼Ī J, Mach├Ī─Źkov├Ī M, Dosoudil M, et al. Intraoperative fluorescence angiography and risk factors of anastomotic leakage in mini-invasive low rectal resections. Surg Endosc 2021;35:5015ŌĆō23.


9. Seike K, Koda K, Saito N, Oda K, Kosugi C, Shimizu K, et al. Laser Doppler assessment of the influence of division at the root of the inferior mesenteric artery on anastomotic blood flow in rectosigmoid cancer surgery. Int J Colorectal Dis 2007;22:689ŌĆō97.


10. Komen N, Slieker J, de Kort P, de Wilt JH, van der Harst E, Coene PP, et al. High tie versus low tie in rectal surgery: comparison of anastomotic perfusion. Int J Colorectal Dis 2011;26:1075ŌĆō8.



11. Trottier DC, Friedlich M, Rostom A. The use of endoscopic hemoclips for postoperative anastomotic bleeding. Surg Laparosc Endosc Percutan Tech 2008;18:299ŌĆō300.


12. Zhong QH, Liu ZZ, Yuan ZX, Ma TH, Huang XY, Wang HM, et al. Efficacy and complications of argon plasma coagulation for hemorrhagic chronic radiation proctitis. World J Gastroenterol 2019;25:1618ŌĆō27.



13. Atabek U, Pello MJ, Spence RK, Alexander JB, Camishion RC. Arterial vasopressin for control of bleeding from a stapled intestinal anastomosis: report of two cases. Dis Colon Rectum 1992;35:1180ŌĆō2.

- TOOLS
-
METRICS

- Related articles in ACP
-
Genotypic and Phenotypic Characteristics of Hereditary Colorectal Cancer2021 December;37(6)
Efficacy and Safety of Laparoscopic Hartmann Colostomy Reversal 2018 December;34(6)
Safety of Early Postoperative Feeding after Elective Colorectal Surgery.1998 September;14(3)
Clinical Features of Intestinal Obstruction after Colorectal Surgery.2003 December;19(6)







