- Search
Abstract
Purpose
Clostridium difficile (C. difficile)-associated colitis, a known complication of colon and rectal surgery, can increase perioperative morbidity and mortality, leading to increased hospital stay and costs. Several contributing factors, including advanced age, mechanical bowel preparation, and antibiotics, have been implicated in this condition. The purpose of this study was to determine the clinical features of and factors responsible for C. difficile-associated colitis after colorectal cancer surgery.
Methods
The medical records of patients who had undergone elective resection for colorectal cancer from January 2008 to April 2010 were reviewed. Cases that involved procedures such as transanal excision, stoma creation, or emergency operation were excluded from the analysis.
Results
Resection with primary anastomosis was performed in 219 patients with colorectal cancer. The rate of postoperative C. difficile-associated colitis was 6.8% in the entire study population. Preoperative metallic stent insertion (P = 0.017) and aged sixty and older (≥ 60, P = 0.025) were identified as risk factors for postoperative C. difficile-associated colitis. There were no significant differences in variables such as preoperative oral non-absorbable antibiotics, site of operation, operation procedure, and duration of prophylactic antibiotics.
Conclusion
Among the potential causative factors of postoperative C. difficile-associated colitis, preoperative metallic stent insertion and aged sixty and older were identified as risk factors on the basis of our data. Strategies to prevent C. difficile infection should be carried out in patients who have undergone preoperative insertion of a metallic stent and are aged sixty and older years.
Clostridium difficile (C. difficile)-associated colitis is acute infectious colitis caused by the toxin of the abnormally proliferated anaerobe gram positive bacteria C. difficile. The clinical features of C. difficile-associated colitis are diverse, ranging from mild diarrhea to pseudomembranous-colitis-induced fever, abdominal pain, abdomen distention, leukocytosis, and fulminant colitis causing hemorrhage and necrosis [1-3].
As risk factors for C. difficile-associated colitis, old age, impaired immune function, intestinal retention, renal failure, chemotherapy, and surgery itself in the gastrointestinal system have been reported [4, 5]. Particularly, the normal bacterial flora in the intestine has been shown to be destroyed by the use of antibiotics; thus, C. difficile forms colonies in the intestine and causes C. difficile-associated colitis. In addition, preoperative mechanical bowel preparation performed prior to surgery for colorectal cancer, the use of oral antibiotics and prophylactic intravenous antibiotics, and the proctocolectomy itself change the normal bacterial flora in the intestine; hence, they have been shown to be risk factors. Nonetheless, systematized reports are rare [6-9]. Therefore, we examined the clinical features of and the risk factors for C. difficile-associated colitis after colorectal cancer surgery.
The study was conducted on 219 patients diagnosed as having colorectal cancer and treated with a radical resection and primary anastomosis at our hospital from January 2008 to April 2010. Patients without a primary anastomosis, such as those with stomy creation and transanal operation, were excluded, and patients on whom emergency surgery without bowel preparation had been performed were excluded. In all patients, mechanical bowl preparation was performed 1 or 2 days prior to surgery with either 90 mL of Fleet phospho-soda® (sodium phosphate; Merck Frosst Canada Inc., Kirkland, QU, Canada) or 4 L of Colyte-F® (polyethylene glycol, PEG; Taejoon Pharm, Seoul, Korea). From January 2008 to February 2009, patients were treated with oral antibiotics prior to surgery; after that date, oral antibiotics were not used. As oral antibiotics, one day before surgery, at 12 pm, 6 pm, and 10 pm, kanamycin and metronidazole were taken. As prophylactic intravenous antibiotics, in all patients, the second generation cephalosporin antibiotic Pacetin® (cefoxitin; Choongwae Pharm Co., Seoul, Korea) 2 g was injected intravenously 30 minutes before skin incision. For the maintenance of intravenous antibiotics, according to intraoperative findings, antibiotics were empirically administered from one injection to up to for 5 days. Patients were diagnosed as having C. difficile-associated colitis when the clinical symptoms of watery diarrhea more than 3 times per day, fever, abdominal pain, abdominal distention, leukocytosis, and positive C. difficile toxin were present.
For statistical analysis, the SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA) statistics program was used. For the comparative analysis of variables, the chi-square test or the Fisher's exact test was applied. As statistical significance, cases with P-values lower than 0.05 were considered to be significant.
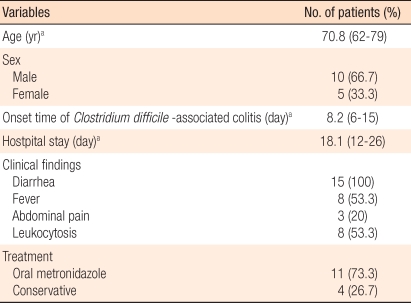
The mean age of the 219 colorectal cancer patients was 64.7 years (range, 28 to 84 years), 120 patients were male, and 99 patients were female. The primary lesion site was the right side colon in 71 patients (32.5%), the left side colon in 103 patients (46.9%), and the rectum in 45 patients (20.6%). Among patients who underwent surgery for colorectal cancer, 15 patients were diagnosed as having C. difficile-associated colitis, and the incidence was 6.8%.
The mean age of patients diagnosed as having C. difficile-associated colitis was 70.8 years (range, 62 to79 years), and the ratio of males to females was 2:1 (Table 1). The mean interval from surgery to the manifestation of colitis symptoms was 8.2 days (range, 6 to 15 days). The mean hospital stay of these patients was 18.1 days (range, 12 to 26 days). As clinical features, 5 patients (33.3%) showed only diarrhea without systemic symptoms, and 10 patients (66.7%) presented with fever, abdominal pain, and leukocytosis.
As treatments for C. difficile-associated colitis, administration of oral metronidazole was used for 11 patients (73.3%), and the remaining 4 patients were not treated with oral antibiotics; only conservative treatments were performed. None of the patients developed complications.
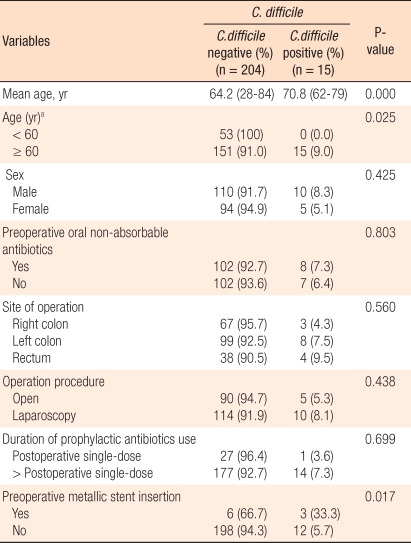
Comparing C. difficile-associated colitis patients and the control patients, gender was not statistically different (Table 2). The mean age of the control group was 64.2 years, and it was significantly higher in the colitis patient group (P = 0.000). In patients younger than 60 years, C. difficile-associated colitis was not observed. The incidence of C. difficile-associated colitis in patients older than 60 years was higher, and this difference was statistically significant (P = 0.025).
Prior to surgery, oral antibiotics were used in 110 patients (50.2%), and the development of C. difficile-associated colitis for those patients was not significantly different from that for the control group. Regarding the primary lesion, C. difficile-associated colitis developed slightly more in surgery involving the rectum, but that difference was not statistically significant. Regarding surgical methods, no differences between laparoscopic surgery and open were detected. The duration of the use of prophylactic intravenous antibiotics was examined, and no significant difference between the patient group and the control group was detected. In 9 patients (4.1%), because of the obstruction caused by colorectal cancer, surgery was performed after inserting a stent, and the stent insertion was shown to be a significant risk factor for the development of colitis (P = 0.017). A multivariate analysis was performed, and the insertion of a stent due to an obstruction by colorectal cancer was shown to be an independent risk factor (P = 0.001).
C. difficile-associated colitis is colitis caused by the toxin of the anaerobic gram positive bacteria C. difficile, it usually occurs as a nosocomial infection, and the incidence has been reported to be 2.3-7.8% [1, 10, 11]. It may develop in patients who have undergone surgery. It particularly shows a high incidence in patients who have undergone surgery involving the gastrointestinal tract, and the incidence after a colorectal resection has been reported to be up to 21% [5]. The incidence of C. difficile-associated colitis after colorectal resection, as assessed in our study, was 6.8%.
Clinical courses of infection caused by C. difficile are from asymptomatic carriers to patients with diarrhea, pseudomembranous colitis, or fulminant colitis such as toxic megacolon [12]. In many patients, it is asymptomatic or limited to mild diarrhea; 25% of the cases show pseudomembranous colitis, and it progresses to fulminant colitis in 1-3% of the cases [2, 13]. The diarrhea pattern has been reported to be primarily watery diarrhea rather than bloody diarrhea, and it develops within 48-72 hours after infection. In our study, the mean interval from surgery to the manifestation of colitis symptoms was 8.2 days (range, 6 to 15 days).
In fulminant colitis cases, although rare, bowel perforation, toxic megacolon, and other complications may develop, and most patients experience associated abdominal pain, abdominal distention, hypotension, fever, and leukocytosis. As its treatment, surgery is required, and mortality reaches 35-80%; thus, rapid diagnosis and treatment are required [14]. Rubin et al. [2] reported that in C. difficile-associated colitis patients, 3% of the patients show severe clinical courses, and they require intensive treatment and may die. Symptoms more frequently presented in cases of severe C. difficile-associated colitis are abdominal pain, abdominal distention, and leukocytosis. In addition, as risk factors for severe colitis, tumors, chronic obstructive pulmonary disease, impairment of immune function, anti-peristaltic drugs, and renal impairment have been reported. Nonetheless, diarrhea, abdominal pain, and fever are symptoms that frequently develop after surgery for colorectal cancer due to various causes, and their differentiation is difficult. In our cases, 5 patients (33.3%) developed diarrhea without systemic symptoms, and 10 patients (66.7%) had systemic symptoms, such as fever, abdominal pain, and leukocytosis; nonetheless, none of the patients showed severe colitis. As treatments for patients showing watery diarrhea, the use of all antibiotics was terminated, and a stool test was performed; for patients positive for C. difficile toxin, treatments were initiated.
As risk factors for C. difficile-associated colitis, old age, impaired immune function, intestinal retention, renal failure, chemotherapy, gastrointestinal surgery, and the use of antibiotics have been reported [4, 5, 15], and recently, proton pump inhibitors and quinolone antibiotics have been reported [16, 17]. In addition, preoperative mechanical bowel preparation for colorectal cancer, the use of oral antibiotics, intravenous antibiotics, and the proctocolectomy itself have been reported to change the normal bacterial flora of the intestine and are thus risk factors for C. difficile-associated colitis [6-9].
Since Clarke et al. [7] reported in 1977 that in colorectal surgery, mechanical bowel preparation and the use of antibiotics during surgery reduced bacterial infection and consequently postsurgical infectious complications, mechanical bowel preparation and prophylactic antibiotics have been used until now. Recently, however, the necessity of the use of mechanical bowel preparation and the use of oral antibiotics has been questioned [9, 18]. Most studies regarding mechnical bowel preparation and the use of antibiotics have looked at the infectious complications and anastomotic leakage, but studies on C. difficile-associated colitis are rare. Wren et al. [19] reported that during colorectal surgery, the use of oral antibiotics induced a change in the normal bacterial flora of the large intestine, thus increasing the incidence of C. difficile-associated colitis. In our study, mechanical bowel preparation was performed on all patients, oral antibiotics were used in 110 patients (50.2%), and no oral antibiotics were used in the remaining 109 patients (49.8%). No significant differences in the development of colitis were observed between patients receiving oral antibiotics and those not receiving oral antibiotics.
Age has been reported to be an independent risk factor for C. difficile-associated colitis in many studies. McFarland et al. [20] reported in a prospective study that age was an important risk factor. Similarly, McDonald et al. [21] reported that according to age, differences in the development of C. difficile-associated colitis were observed; particularly, in patients older than 65 years old, the incidence of C. difficile infection was high. In our study, age older than 60 years was shown to be a significant risk factor for C. difficile-associated colitis. However, in the multivariate analysis, age was not an independent risk factor.
The use of antibiotics, particularly the third-generation cephalosporin antibiotics, has been reported to be an important risk factor for C. difficile infection, and to limit their use is the most effective measure to prevent C. difficile infection [22]. In addition, penicillin, aminoglycoside, clindamycin, fluoroquinolone, and other antibiotics have been reported to be associated with C. difficile infection [23, 24]. Kent et al. [5] reported that among several antibiotics, the use of cefoxitin was a risk factor for C. difficile-associated colitis. Metzger et al. [25] reported that the incidence of C. difficile-associated diarrhea was high in the elderly, but no significant differences among antibiotics were detected. In our study, only the second-generation cephalosporin antibiotic Pacetin® was used in all patients; thus, no analysis of the effect of the type of antibiotic could be performed.
The development of C. difficile-associated colitis has been reported to be generally related to long-term antibiotics treatment. However, infection after using an antibiotic only once or for a short term has been reported. Hence, the duration of the exposure to antibiotics is a subject of controversy. Park et al. [26] reported that in patients who underwent gastrointestinal surgery, the duration of the use of aminoglycoside antibiotics was an independent risk factor for the development of pseudomembranous colitis. In our study, the effect of the duration of use of antibiotics was analyzed by dividing the duration of use of prophylactic antibiotics based on use once after surgery, and no significant differences were observed. Generally, the use of prophylactic antibiotics once after surgery is sufficient, and the duration of its use is determined by the half-life of the antibiotic, the operation time, and the occurrence of contamination during surgery. Nonetheless, at our hospital, because of the burden of infectious complications, the duration of actual use is extended to up to 5 days.
Colorectal surgery has been reported to be a risk factor for C. difficile-associated colitis in many studies. Keighley et al. [27] reported that among patients receiving gastrointestinal surgery, the incidence of C. difficile-associated colitis was high in patients undergoing a colorectal resection. Kent et al. [5] examined 374 surgery patients and reported that the incidence of C. difficile-associated colitis was 5.6%. They reported that among them, 21% of the patients who underwent colorectal surgery and 29% of the patients who underwent surgery for small bowel and large bowel obstruction, C. difficile-associated colitis developed; in addition, intestinal retention was an independent risk factor. In our study, insertion of stents for colorectal cancer obstruction prior to surgery was confirmed to be an independent risk factor for the development of C. difficile-associated colitis. Obstruction of the large bowel, as well as the small bowel, is thought to cause a change in the normal bacterial flora of the intestine, which has an effect on the development of C. difficile-associated colitis. In the analysis according to the site of the colorectal resection, the tendency was that in patients undergoing surgery for rectal cancer, the incidence of C. difficile-associated colitis was slightly high, but no statistical significance was shown.
In regard to treatments for C. difficile-associated colitis, until now, metronidazole and vancomycin have been reported to be effective therapeutics [28]. However, according to recent studies, the treatment efficacy of metronidazole, known to be a first-line treatment therapeutic, is worse than before. The reasons have been reported to be the aging of patients, an increase in the severity of underlying diseases, and the use of broad spectrum antibiotics, such as quinolone. In our cases, as treatments for C. difficile-associated colitis, oral metronidazole was administered to 11 patients (73.3%), and for the remaining 4 patients, no antibiotics were administered; only conservative treatments were performed. None of patients progressed to pseudomembranous colitis or fulminant colitis, and all patients were cured without complications.
In conclusion, clinical symptoms of C. difficile-associated colitis, which develops in high incidence in patients undergoing colorectal surgery, were diverse, and it was difficult to differentiate the patients (for C. difficile-associated colitis) based solely on symptoms. In addition, C. difficile-associated colitis may prolong the hospital stay after surgery and increase cost, and it could influence morbidity and mortality. Thus, early diagnosis and treatment of C. difficile-associated colitis is considered to be important.
C. difficile-associated colitis occurs at high rates after colorectal surgery. Risk factors are the insertion of stents prior to surgery and an age older than 60 years. Therefore, in patients undergoing surgery for colorectal cancer, if the symptoms of diarrhea, abdominal pain, fever, and leukocytosis are observed, keeping in mind the possibility of C. difficile-associated colitis, rapid diagnosis and treatments should be achieved. Particularly, for elderly patients older than 60 years and patients who had stents inserted prior to surgery for large bowel obstruction, strategies to prevent C. difficile infection are required.
Notes
References
1. Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N Engl J Med 1994;330:257–262. PMID: 8043060.


2. Rubin MS, Bodenstein LE, Kent KC. Severe Clostridium difficile colitis. Dis Colon Rectum 1995;38:350–354. PMID: 7720439.


3. Triadafilopoulos G, Hallstone AE. Acute abdomen as the first presentation of pseudomembranous colitis. Gastroenterology 1991;101:685–691. PMID: 1860633.


4. Hurley BW, Nguyen CC. The spectrum of pseudomembranous enterocolitis and antibiotic-associated diarrhea. Arch Intern Med 2002;162:2177–2184. PMID: 12390059.


5. Kent KC, Rubin MS, Wroblewski L, Hanff PA, Silen W. The impact of Clostridium difficile on a surgical service: a prospective study of 374 patients. Ann Surg 1998;227:296–301. PMID: 9488530.



6. Groner JI, Edmiston CE Jr, Krepel CJ, Telford GL, Condon RE. The efficacy of oral antimicrobials in reducing aerobic and anaerobic colonic mucosal flora. Arch Surg 1989;124:281–284. PMID: 2919961.


7. Clarke JS, Condon RE, Bartlett JG, Gorbach SL, Nichols RL, Ochi S. Preoperative oral antibiotics reduce septic complications of colon operations: results of prospective, randomized, double-blind clinical study. Ann Surg 1977;186:251–259. PMID: 889372.



8. Lindsey JT, Smith JW, McClugage SG Jr, Nichols RL. Effects of commonly used bowel preparations on the large bowel mucosal-associated and luminal microflora in the rat model. Dis Colon Rectum 1990;33:554–560. PMID: 2361422.


9. Zmora O, Pikarsky AJ, Wexner SD. Bowel preparation for colorectal surgery. Dis Colon Rectum 2001;44:1537–1549. PMID: 11598488.


10. Brown E, Talbot GH, Axelrod P, Provencher M, Hoegg C. Risk factors for Clostridium difficile toxin-associated diarrhea. Infect Control Hosp Epidemiol 1990;11:283–290. PMID: 2373850.


11. McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med 1989;320:204–210. PMID: 2911306.


12. Freeman HJ. Recent developments on the role of Clostridium difficile in inflammatory bowel disease. World J Gastroenterol 2008;14:2794–2796. PMID: 18473400.



13. Longo WE, Mazuski JE, Virgo KS, Lee P, Bahadursingh AN, Johnson FE. Outcome after colectomy for Clostridium difficile colitis. Dis Colon Rectum 2004;47:1620–1626. PMID: 15540290.


14. Greenstein AJ, Byrn JC, Zhang LP, Swedish KA, Jahn AE, Divino CM. Risk factors for the development of fulminant Clostridium difficile colitis. Surgery 2008;143:623–629. PMID: 18436010.


15. Surawicz CM, McFarland LV. Pseudomembranous colitis: causes and cures. Digestion 1999;60:91–100. PMID: 10095149.


16. Kazakova SV, Ware K, Baughman B, Bilukha O, Paradis A, Sears S, et al. A hospital outbreak of diarrhea due to an emerging epidemic strain of Clostridium difficile. Arch Intern Med 2006;166:2518–2524. PMID: 17159019.


17. Muto CA, Pokrywka M, Shutt K, Mendelsohn AB, Nouri K, Posey K, et al. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol 2005;26:273–280. PMID: 15796280.


18. Lau WY, Chu KW, Poon GP, Ho KK. Prophylactic antibiotics in elective colorectal surgery. Br J Surg 1988;75:782–785. PMID: 3167527.


19. Wren SM, Ahmed N, Jamal A, Safadi BY. Preoperative oral antibiotics in colorectal surgery increase the rate of Clostridium difficile colitis. Arch Surg 2005;140:752–756. PMID: 16103284.


20. McFarland LV, Surawicz CM, Stamm WE. Risk factors for Clostridium difficile carriage and C. difficile-associated diarrhea in a cohort of hospitalized patients. J Infect Dis 1990;162:678–684. PMID: 2387993.



21. McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996-2003. Emerg Infect Dis 2006;12:409–415. PMID: 16704777.



22. Starr JM, Martin H, McCoubrey J, Gibson G, Poxton IR. Risk factors for Clostridium difficile colonisation and toxin production. Age Ageing 2003;32:657–660. PMID: 14600008.



23. Owens RC Jr, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis 2008;46(Suppl 1): S19–S31. PMID: 18177218.



24. McFarland LV, Clarridge JE, Beneda HW, Raugi GJ. Fluoroquinolone use and risk factors for Clostridium difficile-associated disease within a Veterans Administration health care system. Clin Infect Dis 2007;45:1141–1151. PMID: 17918075.



25. Metzger R, Swenson BR, Bonatti H, Hedrick TL, Hranjec T, Popovsky KA, et al. Identification of risk factors for the development of clostridium difficile-associated diarrhea following treatment of polymicrobial surgical infections. Ann Surg 2010;251:722–727. PMID: 20101175.



26. Park BS, Kim JH, Seo HI, Kim HS, Kim DH, Cho HJ, et al. Pseudomembranous Colitis after gastrointestinal operation. J Korean Surg Soc 2009;77:106–112.

27. Keighley MR, Burdon DW, Alexander-Williams J, Shinagawa N, Arabi Y, Thompson H, et al. Diarrhoea and pseudomembranous colitis after gastrointestinal operations: a prospective study. Lancet 1978;2:1165–1167. PMID: 82138.


28. Fekety R. Guidelines for the diagnosis and management of Clostridium difficile-associated diarrhea and colitis. American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 1997;92:739–750. PMID: 9149180.

- TOOLS
-
METRICS

- Related articles in ACP
-
Risk Factors for Incisional Hernia and Parastomal Hernia after Colorectal Surgery2012 December;28(6)
The Risk Factors of
Clostridium difficile Colitis in Colorectal Surgery2010 October;26(5)Risk Factors of a Pulmonary Thromboembolism After Colorectal Surgery2015 October;31(5)