- Search
|
|
Abstract
Purpose
During a laparotomy, the peritoneum is exposed to the cold, dry ambient air of the operating room (20â, 0%â5% relative humidity). The aim of this review is to determine whether the use of humidified and/or warmed CO2 in the intraperitoneal environment during open or laparoscopic operations influences postoperative outcomes.
Methods
A review was performed in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The PubMed, OVID MEDLINE, Cochrane Central Register of Controlled Trials and Embase databases were searched for articles published between 1980 and 2016 (October). Comparative studies on humans or nonhuman animals that involved randomized controlled trials (RCTs) or prospective cohort studies were included. Both laparotomy and laparoscopic studies were included. The primary outcomes identified were peritoneal inflammation, core body temperature, and postoperative pain.
Results
The literature search identified 37 articles for analysis, including 30 RCTs, 7 prospective cohort studies, 23 human studies, and 14 animal studies. Four studies found that compared with warmed/humidified CO2, cold, dry CO2 resulted in significant peritoneal injury, with greater lymphocytic infiltration, higher proinflammatory cytokine levels and peritoneal adhesion formation. Seven of 15 human RCTs reported a significantly higher core body temperature in the warmed, humidified CO2 group than in the cold, dry CO2 group. Seven human RCTs found lower postoperative pain with the use of humidified, warmed CO2.
In recent years, minimally invasive abdominal operations have become prevalent. However, based on pathology and patient factors, open abdominal operations continue to be routinely performed. After open abdominal surgery, commonly encountered complications include postoperative ileus, postoperative infection, wound infection and anastomotic breakdown. Intraoperatively, the bowel is exposed to the ambient air, which is cold and dry relative to the unexposed abdominal environment. The average room temperature in the operating room (OR) is typically 20â, and the average relative humidity is 0%â5%. The OR has negative air ventilation; i.e., clean air is blown in from the ceiling and then out of the OR. The dry and cold air convection causes serosal and peritoneal desiccation [1]. Desiccation results from superficial water loss through diffusion and convective gas movements. When the ambient gas is fully saturated, water loss cannot occur, regardless of the gas movements above the surface. By contrast, if the gas is not fully saturated with water, then convection will be a decisive factor for desiccation. Diffusion alone is a rather slow transfer process, but convection maximizes the evaporation rate by constantly exchanging the âhumidifiedâ gas close to the surface of the intestine with âdryâ ambient gas (the diffusion gradient is maintained at a maximum) [1]. Surgeons who perform a prolonged laparotomy frequently cover the bowel with a warm, wet sponge to moisten the bowel.
Peritoneal desiccation leads to peritoneal inflammation, loss of barrier function and increased risk of infection [1-4], and peritoneal inflammation can lead to postoperative adhesion formation and long-term bowel obstruction [1, 2]. Cooling of the bowel due to exposure can lead to vasoconstriction of splanchnic blood flow to the intestine, which may increase the risk of bowel anastomosis breakdown. Bowel desiccation may also be a factor in delaying the return of bowel function. Previous studies [2-4] have also suggested that desiccation and cooling of the peritoneum from open surgical wounds or the use of cold, nonhumidified CO2 insufflation gas may cause oxidative stress on peritoneal mesothelial cells. Thus, desiccation of the peritoneum has the potential to cause peritoneal inflammation and reduced splanchnic blood flow, with associated long-term consequences. One way of mitigating desiccation is the use of humidified, warmed CO2 gas. CO2 gas is heavier than other components of room air (nitrogen, oxygen) and therefore tends to sink into the abdominal wound rather than drift away. CO2 also maintains heat, thus creating a localized greenhouse effect within the abdominal cavity. Animal studies have also suggested that CO2 pneumoperitoneum has anti-inflammatory properties compared with a standard laparotomy, with a significantly greater increase in anti-inflammatory cytokines (interleukin [IL]-6) [5] and earlier expression of anti-inflammatory cytokines (IL-6) [6]. CO2 pneumoperitoneum allows better regulation of the immune response to local infection (Escherichia coli peritonitis) and has been associated with lower levels of proinflammatory cytokines (IL-1, tumor necrosis factor-Îą [TNF-Îą]), a lower rate of positive blood culture, and lower bacterial counts in peritoneal fluid [6]. These observations may reflect a combination of improved regulation of the immune response, better splanchnic blood flow and less desiccation as a result of CO2 pneumoperitoneum [7]. The aim of this review was to determine whether evidence supporting the benefits associated with the use of humidified, warmed CO2 during abdominal surgery can be found in the literature.
A comprehensive literature search and review was performed in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [8]. Two authors (JYC, AK) performed the literature search using the databases PubMed, OVID MEDLINE, Cochrane Central Register of Controlled Trials and Embase (1980â2016 October). The key search terms included the following: âcarbon dioxide,â âhumidified,â âlaparotomy,â âsurgical procedures, minimally invasive,â âpneumoperitoneum,â âlaparoscopy,â âtemperature,â âhelium,â âoutcomes,â âinflammation,â and âpostoperative pain.â
Comparative studies, including randomized controlled trials (RCTs) and prospective cohort studies, were included. Both laparotomy and laparoscopic studies were reviewed; laparoscopic studies that compared the effects of humidified, warmed CO2 pneumoperitoneum with those of cold, dry CO2 pneumoperitoneum were included because cold, dry CO2 pneumoperitoneum is analogous to and mimics the environment in the operating theatre. Studies of humans and nonhuman animals were included. For human subject studies, a sample size of at least 20 patients was a prerequisite for inclusion. For work conducted with non-human animals, a minimum of 10 animals per treatment group was considered necessary for inclusion. A lower minimum sample size was acceptable for nonhuman animal studies because such studies tend to be more homogenous and better controlled than human studies.
Potentially eligible studies were selected based on their titles and abstracts. The full texts of these publications were obtained and reviewed to confirm the eligibility of each study for inclusion in the review. The reference lists of included and excluded articles were searched to identify any additional relevant articles. All publications related to each individual RCT were obtained along with the trial protocol, if available, in electronic format. The primary outcomes identified were peritoneal inflammation, core body temperature, postoperative pain, wound infection and tumor growth. An assessment of the quality of the evidence provided in the various studies was determined for each study by using the Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence system [9]. Given the significant heterogeneity in the outcome measures, no formal meta-analysis was applied, and the study results are presented individually in tabular format.
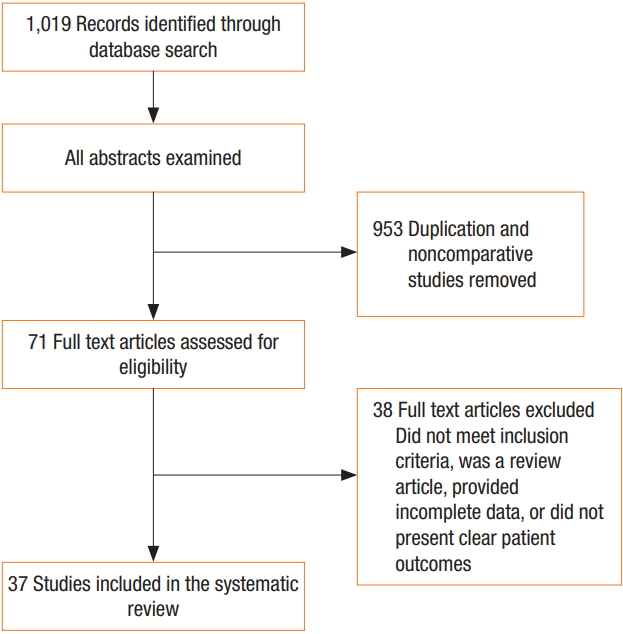
The literature search identified 37 articles that met the inclusion criteria for analysis (Fig. 1), including 30 RCTs, 7 nonrandomized cohort studies, 23 human studies, and 14 animal studies. The animal model studies examined the effects of pneumoperitoneum under various conditions (warmed/humidified CO2 vs. cold/dry CO2 vs. ambient air vs. helium). In human studies, the effects of various pneumoperitoneum conditions (warmed/humidified CO2 vs. cold/dry CO2 vs. air) in different operative settings were examined (bariatric surgery, colorectal surgery, gynecological surgery, and hepatobiliary surgery).
Eight studies compared the effects of warmed, humidified CO2 insufflation vs. cold, dry CO2 insufflation on peritoneal morphology, inflammation and adhesion formation (Table 1). Six studies were animal-based, and 2 were human studies. The 2 human studies examined the effects in patients undergoing a laparoscopic cholecystectomy or a laparoscopic colectomy [10, 11]. Brokelman et al. [10] found that the peritoneal plasminogen activator inhibitor-1 (PAI-1) level was ten times higher in peritoneal biopsies of patients who received cold gas insufflation than it was in patients who received warmed, humidified CO2. Elevated PAI-1 levels can lead to lower levels of fibrinolysis and increased fibrin deposition, resulting in increased peritoneal adhesion formation [12]. An analysis of abdominal drain fluid 20 hours postoperatively found no difference in the levels of inflammatory cytokines (IL1, 6, 8, 10, and TNF-Îą) between the 2 groups [11].
The largest animal study employed 150 rats. The group that received cold, dry gas pneumoperitoneum had more intense peritoneal injury, with intra-abdominal adhesions, than the group that received warmed, humidified gas pneumoperitoneum [13]. Three smaller animal studies [14-16] found no difference in peritoneal inflammation between the 2 pneumoperitoneum groups; however, one found that cold, dry gas insufflation resulted in greater infiltration of the peritoneum by lymphocytes and desquamation of the mesothelial cells, with exposure of the underlying basement membranes [17]. One animal study [18] compared the effects of CO2 pneumoperitoneum with those of air pneumoperitoneum and found that the air pneumoperitoneum group had higher levels of infiltration of the peritoneum by inflammatory cells than the CO2 pneumoperitoneum group, with a threefold increase in the number of polymorphoneutrophils (PMNs) and lower PMN apoptosis rates. Collectively, these studies suggest that insufflation with cold, dry gas to create the pneumoperitoneum is associated with greater peritoneal injury, increased adhesion formation and greater recruitment of inflammatory cells; however, no difference in peritoneal cytokine levels was observed between treatment groups.
The surface area of the peritoneal cavity is equivalent to that of the external body, 1â2 m2 [2]. Approximately 10% of cardiac output is routed through the splanchnic system. The peritoneal cavity has the potential for extensive heat exchange and resultant morbidity due to hypothermia [19-21]. Twenty studies examined the effects of humidified, warmed CO2 on core body temperature (Table 2). Three studies were animal-based, and 17 studies were human-based. Among the 17 available human studies, 15 were RCTs, and 2 were nonrandomized prospective studies. The total number of patients in the RCTs was 1,014. The studies all involved laparoscopic operations. Many of the studies had small sample sizes, and only 7 studies had more than 30 patients in each arm [22-28]. Seven studies [19, 23, 27, 29-32] found significantly higher core body temperatures in the treatment group (humidified, warmed CO2 group) than in the control group (cold, dry CO2) whereas nine studies did not find any significant difference [22, 24-26, 28, 33-36].
The largest human-based RCT involved 195 patients who had undergone a laparoscopic appendectomy [26]. The study found no difference in core body temperature between the treatment group and the control group. However, the next-largest human RCT, based on 148 patients who had undergone a laparoscopic cholecystectomy, found higher core temperatures in the warmed, humidified CO2 group (37.07â vs. 36.85â, P = 0.01) [27]. Another study found that the core temperature decreased in both the treatment and the control groups; however, the decrease was greater in the treatment group (0.7â to 0.3â and 0.3â to 0.1â, respectively) [37].
The largest animal study was conducted with 150 rats [13]; a decrease in core body temperature of between 2.3â and 3.1â was observed in the control group treated with cold/dry CO2 pneumoperitoneum whereas the core body temperature of the humidified/warmed CO2 group increased by 1.3â. Another animal study examined the effects of 4 different combinations of pneumoperitoneum conditions: cold/dry, cold/humidified, warm/dry, and warm/humidified [15]. Conditions that were either cold or dry resulted in a significant decrease in core body temperature. By contrast, warmed/humidified pneumoperitoneum resulted in an increase in core body temperature of 2.4â (P = 0.031) [15]. Bessell et al. [38] suggested that the humidity of the insufflated CO2 gas was more important than its temperature in maintaining core body temperature. Their study compared the use of dry-warm CO2 with that of dry-cold CO2 and found that both resulted in a significant drop in core body temperature. The provision of warmed, rather than cold, insufflated gas conferred no protection against changes in core temperature during laparoscopic surgery due to the small amount of heat required to warm the gas to body temperature. The study suggested that a greater amount of body heat is required to saturate the insufflated gas. Most of the hypothermic effect they observed was due to this saturation and could be minimized by humidifying the gas.
While some evidence exists that warmed, humidified CO2 results in better control of core body temperature than cold, dry CO2, this outcome has not been shown universally. Overall, the effect on core temperature appears to be more pronounced in larger studies than in smaller studies, suggesting that the smaller-scale studies were underpowered.
Sixteen studies compared the effects of humidified, warmed CO2 with those of cold, dry CO2 on postoperative pain (Table 3). All of these studies were human RCTs, with a total of 1,223 patients. Pain was measured using a combination of the visual analogue score (VAS), verbal rating scale (VRS), morphine equivalent daily dose (MEDD), analogue pain score, Likert Scale and total analgesia requirement. Nine studies (with a total of 602 patients) found no difference in postoperative pain [11, 22, 23, 25, 26, 30, 32, 34, 36] whereas seven studies (with a combined total of 621 patients) found that pain was significantly lower with the use of humidified, warmed CO2 [27-29, 33, 39-41].
The largest RCT, which was conducted by Yu et al. [26], revealed no difference in MEDD and VAS scores between the warmed, humidified CO2 pneumoperitoneum group and the cold, dry CO2 pneumoperitoneum group among patients who had undergone a laparoscopic appendectomy. However, a laparoscopic appendectomy is a minor operation, and whether the results of such a study can be justifiably compared with those of other studies involving major surgeries is questionable. The next-largest human RCT, which included 148 patients who had undergone a laparoscopic cholecystectomy, found that postoperative pain measured by using the VAS at day 0 was significantly lower in the warmed, humidified CO2 group than it was in the cold, dry CO2 group [27].
Similarly, Mouton et al. [33] determined that warmed, humidified CO2 resulted in less perioperative pain, with improved pain noted for up to 10 days postoperatively, and Farley et al. [23] observed a similar benefit at postoperative day 14. Thus, while some evidence showing that the use of warmed, humidified CO2 reduces postoperative pain exists, the overall evidence is inconclusive.
The CO2 in the peritoneal cavity may be absorbed systemically, with conversion to carbonic acid (H2CO3) (Table 4). This buffering effect may decrease pH both locally (within the peritoneum) and systemically depending on the intrinsic carbonic acid levels. Consistent with this possibility, an animal study by BergstrĂśm et al. [42] found that CO2 pneumoperitoneum resulted in a locoregional decrease in pH (6.4 vs. 7.5, P = 0.001) and a decrease in systemic arterial pH (7.43 vs. 7.49, P = 0.004) compared with insufflation with another gas (helium). Another independent animal study found that increasing the CO2 temperature resulted in a greater systemic arterial concentration of CO2 and a corresponding decrease in pH [43]. A locoregional decrease in pH and an increase in PaCO2 will lead to local vasodilatation, which would be especially beneficial during bowel anastomosis.
A human study by Ozgonul et al. [24] found no difference in arterial pH, PaCO2 level, or HCO3- level between subjects receiving cold CO2 and those receiving warmed CO2. Another human study [44] found that the results of a pulmonary function test at 12 hours were significantly better in the warmed CO2 group than in the cold CO2 group.
Although not included in the review, other interesting effects of humidified, warmed CO2 have been discussed in the literature. These effects were not included in this review because they were either observed in noncomparative studies or in vitro. However, the results of these studies are fascinating and should be mentioned.
First is the effect of humidified, warmed CO2 on tumor migration. Nduka et al. [45] compared the effects of warmed CO2 vs. cold CO2 insufflation on peritoneal tumor growth. Twenty WAG rats were subjected to either cold CO2 or warm CO2 peritoneal insufflation. The rats then received injections of 1Ă105/mL of CC531 colon cancer cells into the peritoneal cavity. After three weeks, the extent of tumor spread and cancer weight were measured, as determined by using the peritoneal cancer index (PCI). Cold CO2 insufflation was associated with a significantly higher PCI score (266) compared with warm CO2 insufflation (151), indicating a greater rate of peritoneal tumor growth and spread in the cold CO2 group (P = 0.025). This study suggested a potential pathophysiological mechanism: peritoneal trauma resulting from cold gas insufflation may be responsible for the greater rate of tumor spread.
Furthermore, Texler et al. [46] examined the spread of tumor cells in warmed, humidified CO2 and cold, dry CO2 environments in vitro. A total of 24 tumor cell cultures were insufflated with CO2 in an airtight environment, and laparoscopic instruments were used to agitate the contents of the bottle. The study found that the use of heated and humidified CO2 with airtight seals around the trocars in vitro (thus mimicking the in vivo laparoscopic intra-abdominal environment) reduced cell deposition on the trocars compared with cold, dry CO2 (P = 0.015). Although not a RCT, a study by Tan [47] found that CO2 pneumoperitoneum inhibited tumor cell growth (TCC) for the first 48 hours in rat models. At high CO2 concentrations (10%â15%), the TCC apoptosis and necrosis rates were 2.8â5.6 times higher than those in the controls without CO2 pneumoperitoneum (P < 0.01).
Second is the effect of warmed, humidified CO2 on local tissue oxygen tension. A 2015 study [48] using 15 Wistar rats found that compared with ambient air, local instillation of humidified, warmed CO2 during a laparotomy significantly increased the local tissue oxygen tension by 96.6% (29.8 mmHg) and the local tissue temperature by 3.0â.
Third is the effect of warmed, humidified CO2 on wound infection. Suggestions have been made that warmed, humidified carbon dioxide on open laparotomy may lead to reductions in both wound infection and intra-abdominal infections because CO2 is bacteriostatic. For many years, CO2 at high concentrations has been used in modified atmosphere packaging to prolong the shelf life of fresh food. The effect of CO2 is especially marked in fresh meat [49]. High concentrations of CO2 have a growth-inhibiting effect on most bacteria, including both aerobes and anaerobes [50, 51]. The inhibitory effect of CO2 is associated with two main mechanisms: suffocation and a CO2-specific effect that acts directly on the bacterial cell [1]. A study by Persson et al. [52] found that at body temperature, the bacterial growth after 4 hours of CO2 exposure was 1/100 that after exposure to air.
Moreover, the use of laminar ultraclean airflow from the ceiling downward to the operating table may actually help convey airborne particles from the surgeons into the operating field. A study [53] found that when a surgeon leans over a wound with such an airflow (which is common), the surgeon increases the risk of airborne wound contamination by 27 fold. Additionally, more than 90% of contaminating bacteria in clean surgical wounds have been found to originate from ambient air [54], and a substantial proportion of these bacteria contaminate the wound directly. CO2 is heavier than air and will therefore sink to the bottom of the laparotomy wound. Surplus CO2 from ongoing insufflation will overflow, and this convective current may help block airborne contamination, as theoretically supported by Stokesâ law, which describes the settling velocity of particles in a gas/liquid. A study [6] in which 10 L/min of CO2 was insufflated into open cardiothoracic wounds found that the rate of direct airborne contamination was reduced by 80%.
Furthermore, the use of CO2 has been found to be beneficial in septicemia. A study by Hanly et al. [55] examined the effect of CO2 pneumoperitoneum on lipopolysaccharide (LPS) septicemia. Two experiments were performed. The first involved randomizing 143 rats to receive either CO2, helium, or air pneumoperitoneum while the control group did not receive pneumoperitoneum. The rats then received an IV injection of endotoxins (LPS). Survival was dramatically higher in the group receiving CO2 insufflation (survival rate, 78%) compared with the groups receiving helium (52%) and air (55%) and compared with the control group (42%), P < 0.05. In the second experiment, 65 rats were administered CO2, helium, or air pneumoperitoneum, and the control group did not receive pneumoperitoneum. All rats underwent a laparotomy, and endotoxins (LPS) were injected intraperitoneally. The survival rate was significantly higher in the CO2 pneumoperitoneum group (85%) than that in the controls (25%), P < 0.05. Cytokine measurements revealed that the IL-10 level was 35% higher in the CO2 pneumoperitoneum group than in the other groups (P < 0.05), and that the TNF-Îą level was 1 of 3 that of the other groups (P < 0.05). This study suggested the presence of direct humoral mediation by CO2, in which the suppression of TNF-Îą release from macrophages by IL-10 may have been responsible for the increased survival. The results of this study are astounding. The possibility that CO2 pneumoperitoneum has a positive impact on survival in septic patients implies that CO2 pneumoperitoneum may actually be beneficial in acutely ill patients with septicemia (e.g., acute abdomen or trauma). In acute settings where laparotomies are performed more frequently, CO2 pneumoperitoneum may still be established and may improve patient survival. This will be discussed further in the sections below.
In this literature review, human and nonhuman animal studies, as well as laparoscopic and laparotomy studies, were examined. Equivocal evidence exists concerning the benefits of warmed, humidified CO2 on the postoperative outcomes of abdominal surgery. Four of eight studies showed that peritoneal inflammation and peritoneal damage were lower in the warmed, humidified CO2 group than in the cold, dry CO2 group. Some evidence also exists that warmed, humidified CO2 results in a more desirable core body temperature (7 of 15 human RCTs) and less postoperative pain (7 of 16 human RCTs). Warmed, humidified CO2 may lead to better postoperative lung function. Studies have found that CO2 pneumoperitoneum leads to a decrease in local peritoneal pH. This decrease, in combination with local warming and the effects of CO2, may lead to vasodilation in splanchnic blood flow. The locally increased splanchnic blood flow will be of benefit for the integrity of colorectal anastomosis.
This study does have several limitations. First, the studies in this review are very heterogeneous. A Cochrane review in 2011 [56] based on 16 human studies found no evidence to support the use of warmed, humidified CO2 gas, with no observed difference in core body temperature, postoperative pain or perioperative outcomes. In 2016, a meta-analysis based on 17 laparoscopic human studies found that the only benefit of warmed, humidified CO2 was a reduction in immediate postoperative pain [57]. In our review, we expanded our scope to include both open/laparoscopic and human/animal studies, as we believe that many of the potential benefits of warmed, humidified CO2 would not otherwise be mentioned; one of these key benefits may be the effect on peritoneal inflammation. Second, potentially eligible studies were identified based on their title and abstracts. During the process, the two authors performing the search were blinded to neither the names of the authors nor the titles of the journals, which could contribute to selection bias.
In conclusion, the ultimate benefit of using warmed, humidified carbon dioxide during abdominal surgery remains to be determined. A need clearly exists for RCTs involving human subjects to examine the potential impacts of the use of warmed, humidified carbon dioxide during abdominal surgery on peritoneal inflammation and adhesion formation.
Table 1.
Effects of humidified, warmed carbon dioxide on peritoneal inflammation
| Study | Study type | Humans/animals | Operation/samples taken | Device used |
Study group |
Control group |
Results | ||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | No. | Treatment | No. | ||||||
| Peng et al. 2009 [13] | RCT | Animals (rats) | Pneumoperitoneum creation (peritoneum/muscle of anterior/upper abdomen harvested) | Self-developed system | Warmed (37°C) + humidified (95% RH) CO2 gas | 75 | Cold (21°C) + dry (<1% RH) CO2 gas | 75 | Cold, dry group: intense peritoneal injury + intraabdominal adhesions |
| Warmed, humidified group: less peritoneal injury, no adhesion | |||||||||
| Brokelman et al. 2008 [10] | RCT | Humans | Laparoscopic cholecystectomy (parietal peritoneal biopsy) | Thermoflator (Karl Storz GmbH & Co., Tuttlingen, Germany) | Warmed (37°C) CO2 gas | 15 | Cold (21°C) CO2 | 15 | Significantly higher PAI (10x) level in the peritoneum of the control group with cold CO2 insufflation |
| Sammour et al. 2010 [11] | RCT | Humans | Elective laparoscopic colectomy (4-mL peritoneal drain fluid) | Insuflow (MR 860, Fisher & Paykel Healthcare, Auckland, New Zealand) | Warmed (37°C) + humidified (98% RH) CO2 | 41 | Standard CO2 (19°C, 0% RH) | 41 | No difference in peritoneal cytokine levels (IL1, 6, 8, 10, TNF-ι) |
| Sammour et al. 2011 [14] | Prospective, non-RCT | Animals (rats) | Pneumoperitoneum creation (biopsies of liver, kidney, pancreas, jejunum) | Insufflator: CO2-OP-Pneu insufflator, (Wisap, Munich, Germany) | Warmed (37°C), humidified (98% RH) CO2 | 10 | Standard (19°C, 0% RH) | 10 | No difference in oxidative stress measures (malondialdehyde-MDA, Protein Carbonl-PC) |
| Humidifier: Insuflow (MR 860, Fisher & Paykel Healthcare) | |||||||||
| Moehrlen et al. 2006 [18] | RCT | Animals (NMRI mice) | Pneumoperitoneum creation (peritoneal lavage sample) | Olympus laparoscopic UHI-1 insufflator (Olympus Volketswil, Volketswil, Switzerland) | CO2 | 9 | Air | 9 | CO2 pneumoperitoneum resulted in less peritoneal inflammation Air resulted in higher PMN recruitment (3Ă), and lower PMN apoptosis rates |
| Hazebroek et al. 2002 [15] | RCT | Animals (rats) | Pneumoperitoneum creation (peritoneal tissue samples from anterior abdominal wall) | MR600 anesthesia respiratory humidifier (Fisher & Paykel Healthcare) | Group 1- Cold (24.9°C), dry (4% RH) CO2 | 12 | No pneumoperitoneum | 12 | No significant morphological difference among the groups |
| Group 2- Cold (24.8°C), humidified (87% RH) CO2 | 12 | ||||||||
| Group 3- Warm (36.9°C), dry (5% RH) CO2 | 12 | ||||||||
| Group 4- Warm (37.1°C), humidified (88% RH) CO2 | 12 | ||||||||
| Erikoglu et al. 2005 [17] | RCT | Animals (rats) | Pneumoperitoneum creation (peritoneal tissue samples) | Datascope GmbH, Passport XG, Bensheim | Warmed (40°C), humidified (98% RH) CO2 | 10 | No pneumoperitoneum | 10 | Greater peritoneal alteration in the cold, dry CO2 group |
| Cold (21°C), dry (2% RH) CO2 | 10 | ||||||||
| Margulis et al. 2005 [16] | RCT | Animals (pigs) | Laparoscopic nephrectomy (peritoneal fluid sample) | Insuflow (MR 860, Fisher & Paykel Healthcare) | Warmed, humidified CO2 | 5 | Cold, dry CO2 | 5 | No difference in serum and peritoneal levels of TNF-Îą, IL-1, IL-6, glucose, and cortisol |
Table 2.
Effects of warmed, humidified carbon dioxide on core body temperature
| Study | Study type | Humans/animals | Operation | Device used |
Study group |
Control group |
Results | ||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | No. | Treatment | No. | ||||||
| Nguyen et al. 2002 [30] | RCT | Humans | Lap Nissen fundoplication | Insuflow (MR 860, Fisher & Paykel Healthcare, Auckland, New Zealand) | Warmed (37°C) + humidified (95% RH) CO2 gas + warming blanket | 10 | Warming blanket | 10 | Intraabdominal T increased by 0.2°C in the study group, but decreased by 0.5°C in the control group after 1.5 hours |
| Difference not significant | |||||||||
| Hamza et al. 2005 [29] | RCT | Humans | Lap Roux-en-Y gastric bypass | Insuflow (MR 860, Fisher & Paykel Healthcare, NZ) | Warmed (37°C) + humidified (95% RH) CO2 gas | 23 | Room temperature (20°C) gas | 21 | Study group showed a significantly higher core body temperature intraoperatively (35.5°C vs. 35.0°C) and at the end of surgery, P = 0.01 |
| Study group also had a significantly lower rate of postoperative shivering (0% vs. 19%) | |||||||||
| Davis et al. 2006 [22] | RCT | Humans | Lap Roux-en-Y gastric bypass | Control- standard CO2 | Group 1- Warmed CO2 | 33 (11 each group) | Standard CO2 | 11 | No difference in core body temperature or humidity |
| Group 1- heated insufflator tube set (Stryker) | Group 2- Humidified CO2 | ||||||||
| Group 2,3- Insuflow (MR 860, Fisher & Paykel Healthcare) | Group 3- Warmed + Humidified CO2 | ||||||||
| Peng et al. 2009 [13] | RCT | Animals (rats) | Laparoscopic insufflation only | Self-developed system | Warmed (37°C) + humidified (95% RH) CO2 gas | 75 | Cold (21°C) + dry (<1% RH) CO2 gas | 75 | Significant decrease in core body temperature in cold, dry CO2 group (decrease of 2.3°C-3.11°C); warmed + humidified CO2 group showed increased temperature by 1.3°C |
| Mouton et al. 1999 [33] | RCT | Humans | Elective laparoscopic cholecystectomy | Modified LINS-1000 Insufflator (Cook Medical Technology, Queensland, Australia) | Warmed (37°C) + humidified (90% RH) CO2 | 20 | Standard CO2 (21°C, 0% RH) | 20 | No difference in core body temperature or humidity |
| Farley et al. 2004 [23] | RCT | Humans | Elective laparoscopic cholecystectomy | Insuflow Filter Heater Hydrator; (Lexion Medical, St Paul, MN, USA) | Warmed (35°C), humidified (95% RH) CO2 | 49 | Standard CO2 | 52 | Core body temperature increased by 0.29°C in humidified, warmed |
| CO2 group and decreased by 0.03°C in standard group, P = 0.01 | |||||||||
| Saad et al. 2000 [34] | RCT | Humans | Elective laparoscopic cholecystectomy | Flow Therme (WISAP, Sauerlach, Germany) | Warmed (37°C) CO2 | 10 | Standard (21°C) CO2 | 10 | No difference in core body temperature |
| Bäcklund et al. 1998 [31] | RCT | Humans | Elective laparoscopic surgery (not specified) | Therme-Pneu Electronic Ltd., Wisap, Germany | Warmed (37°C) CO2 | 13 | Cold (21°C) CO2 | 13 | Warm CO2 group had higher core body temperature (35.8°C vs. 35.4°C, P < 0.05) |
| Warm CO2 group had higher cardiac index intraoperatively (P < 0.05). Warm CO2 group had better urine output (P < 0.05) and lower requirement of mannitol intraoperatively for low urine output | |||||||||
| Nelskylä et al. 1999 [37] | RCT | Humans | Laparoscopic hysterectomy | Thermoflator (Karl Storz, Tuttlingen, Germany) | Warmed (37°C) CO2 | 18 | Cold (24°C) CO2 | 19 | Greater decrease in temperature in the group with warmed CO2 (0.7°C vs. 0.3°C, 0.3°C vs. 0.1°C) |
| Ozgonul et al. 2007 [24] | RCT | Humans | Elective laparoscopic cholecystectomy | H-500 Fluid warmer (Level 1 Technologies, Inc., Rockland, MA, USA) | Warmed (37°C) CO2 | 31 | Cold (21°C) CO2 | 31 | No difference in core body temperature, mean arterial pressure, or heart rate |
| Hazebroek et al. 2002 [15] | RCT | Animals (rats) | Pneumoperitoneum creation | MR600 anesthesia respiratory humidifier (Fisher & Paykel Healthcare) | Group 1- Cold (24.9°C) dry (4% RH) CO2 | 12 | No pneumoperitoneum | 12 | Cold, dry CO2 group: decrease in core body temperature by 1.6°C (P < 0.001) |
| Group 2- Cold (24.8°C), humidified (87% RH) CO2 | 12 | Cold, humidified CO2 group: decrease in core body temperature by 0.3°C (P = 0.011) | |||||||
| Group 3- Warm (36.9°C), dry (5% RH) CO2 | 12 | Warm, dry CO2 group: decrease in core body temperature by 0.9°C (P = 0.031) | |||||||
| Group 4- Warm (37.1°C), humidified (88% RH) CO2 | 12 | Warm, humidified CO2 group: increase in core body temperature by 2.4°C (P = 0.031) | |||||||
| Ott 1991 [19] | Prospective, non-RCT | Humans | Diagnostic laparoscopy | R. Wolf/Weiss insufflator | Warmed (35°C) CO2 | 20 | Cold CO2 | 20 | In the cold CO2 group, a decrease in core body temperature of 0.3°C per 50 L of CO2 used was observed |
| Warmed, humidified group had improved intraoperative nor-mothermia and postoperative pain, and reduced recovery room stay | |||||||||
| Bessell et al. 1995 [38] | RCT | Animals (pigs) | Pneumoperitoneum creation | LINS-1000 insufflator (Cook Medical Technology, Queensland, Australia) | Warmed (30°C) CO2 | 6 | Cold (25°C) CO2 | 6 | No significant temperature difference was observed between animals receiving cold CO2 and those receiving warm CO2 over a 3-hour period |
| Yeh et al. 2007 [35] | Prospective, non-RCT | Humans | Laparoscopic colectomies | Insuflow (MR 860, Fisher & Paykel Healthcare) | Warmed (36°C), humidified (95% RH) CO2 | 20 | Cold (30.2°C), dry (0% RH) | 20 | No significant difference in change in core body temperature |
| Manwaring et al. 2008 [25] | RCT | Humans | Laparoscopic gynecologic procedures | Insuflow (MR 860, Fisher & Paykel Health care) | Warmed (37°C), humidified (100% RH) CO2 | 30 | Cold, dry CO2 | 30 | No difference in core body temperature or recovery room time |
| Champion and Williams 2006 [36] | RCT | Humans | Laparoscopic Roux-en-Y gastric bypass | Insuflow device (Lexion Medical, St Paul, MN, USA) | Warmed (35°C), humidified (95% RH) CO2 | 25 | Cold, dry CO2 | 25 | No difference in core body temperature, operative time, or recovery room time |
| Yu et al. 2013 [26] | RCT | Humans | Laparoscopic appendectomy | Insuflow (MR 860, Fisher & Paykel Healthcare) | Warmed (37°C), humidified (98% RH) CO2 | 97 | Cold (20°Câ21°C), dry (0% RH) CO2 | 98 | No difference in core body temperature |
| Savel et al. 2005 [32] | RCT | Humans | Laparoscopic Roux-en-Y gastric bypass | Insuflow device (Lexion Medical, St. Paul, MN, USA) | Warmed (35°C), humidified (95% RH) CO2 | 15 | Cold, dry CO2 | 15 | No change in core body temperature in the cold, dry CO2 group |
| In the humidified, warmed CO2 group, core body temperature increased from 35.8°C to 36.2°C (P = 0.004) | |||||||||
| Klugsberger et al. 2014 [27] | RCT | Humans | Laparoscopic cholecystectomy | Optitherm device (Storz, Tuttlingen, Germany) | Warmed, humidified CO2 | 81 | Cold, dry CO2 | 67 | Higher core body temperature in the warmed, humidified CO2 group (37.07°C vs. 36.85°C, P = 0.01) |
| Herrmann and De Wilde 2015 [28] | RCT | Humans | Laparoscopic assisted vaginal hysterectomy | Insuflow (MR 860, Fisher & Paykel Healthcare) | Warmed (37°C), humidified (98% RH) CO2 | 48 | Cold (20°Câ21°C), dry (0% RH) CO2 | 49 | No difference in core body temperature |
Table 3.
Effects of warmed, humidified carbon dioxide on postoperative pain in humans
| Study | Study type | Humans/animals | Operation | Device used |
Study group |
Control group |
Results | Method of measuring pain | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | No. | Treatment | No. | |||||||
| Nguyen et al. 2002 [30] | RCT | Humans | Lap Nissen fundo-plication | Insuflow device (Lexion Medical, St. Paul, MN, USA) | Heated + humidified CO2 gas + warming blanket | 10 | Warming blanket | 10 | No significant difference | VAS |
| Hamza et al. 2005 [29] | RCT | Humans | Lap Roux-en-Y gastric bypass | Insuflow device (Lexion Medical) | Heated + humidified CO2 gas | 23 | Room temperature gas | 21 | Maximum VRS and morphine consumption significantly lower in study group | 11-point VRS |
| Davis et al. 2006 [22] | RCT | Humans | Lap Roux-en-Y gastric bypass | Control- standard CO2 | Group 1-heated CO2 | 33 (11 each group) | Standard CO2 | 11 | No difference in postoperative pain | VAS |
| Group 1- heated insufflator tube set (Stryker) | Group 2-humidified CO2 | |||||||||
| Group 2,3- Insuflow (MR 860, Fisher & Paykel Healthcare, Auckland, New Zealand) | Group 3-heated+ humidified CO2 | |||||||||
| Sammour et al. 2010 [11] | RCT | Humans | Elective laparoscopic colonic resections | Insuflow (MR 860, Fisher & Paykel Healthcare) | Warmed (37°C) + humidified (98% RH) CO2 | 41 | Standard CO2 (19°C, 0% RH) | 41 | No difference in postoperative pain | (1) VAS |
| (2) MEDD | ||||||||||
| Mouton et al. 1999 [33] | RCT | Humans | Elective laparoscopic cholecystectomy | Modified LINS-1000 Insufflator (Cook Medical Technology, Queensland, Australia) | Warmed (37°C) + humidified (90% RH) CO2 | 20 | Standard CO2 (21°C, 0% RH) | 20 | Humidified + warmed CO2 group had significantly less postoperative pain at 6 hours, at 1st, 2nd, 3rd day postoperatively and on follow-up on day 10 | Analogue pain score |
| Mean time to return to normal activity was significantly lower in warmed, humidified group (5.9 days vs. 10.9 days) | ||||||||||
| Farley et al. 2004 [23] | RCT | Humans | Elective laparoscopic cholecystectomy | Insuflow device (Lexion Medical) | Warmed (35°C), humidified (95% RH) CO2 | 49 | Standard CO2 | 52 | No difference in postoperative pain during admission | (1) Likert Scale (010) |
| However, significant difference in pain on follow-up at week 2 (Likert Scale 1.0 vs. 0.3, P = 0.02) | (2) Morphine Equivalent Score (use of analgesia) | |||||||||
| Saad et al. 2000 [34] | RCT | Humans | Elective laparoscopic cholecystectomy | Flow Therme, (WISAP, Sauer-lach, Germany) | Warmed (37°C) CO2 | 10 | Standard (21°C) CO2 | 10 | No difference in postop pain (visual analogue score + analgesia usage) | (1) VAS |
| (2) Postoperative ibuprofen usage | ||||||||||
| Beste et al. 2006 [39] | RCT | Humans | Laparoscopic gynecological procedures: tubal ligation, salpingo-oo-phorectomy, cystectomy, ablation of endometriosis, adhesiolysis, che-mopertubation | Insuflow device (Lexion Medical) | Warmed, humidified CO2 | 47 | Warmed, dry CO2 | 42 | Humidified CO2 reduced postoperative pain and requirements for analgesia | Total morphine equivalent |
| Kissler et al. 2004 [40] | RCT | Humans | Laparoscopic gynecological procedures | Laparo-CO2-pneu 2232 (Wolf, Knit-tlingen, Germany) | Warmed, humidified CO2 | 30 | Cold, dry CO2 | - | Significant differences in postoperative pain and analgesia requirements | (1) Total analgesia requirement |
| Warmed, dry CO2 | 30 | 30 | Non-significant tendency towards less pain and higher patient satisfaction in patients who received cold, dry CO2 | (2) VAS | ||||||
| (3) Patient satisfaction | ||||||||||
| Manwaring et al. 2008 [25] | RCT | Humans | Laparoscopic gynecological procedures | Insuflow (MR 860, Fisher & Paykel Healthcare) | Heated (37°C), humidified (100% RH) CO2 | 30 | Cold, dry CO2 | 30 | No difference in postoperative pain or analgesia requirements | VAS |
| Champion and Williams 2006 [36] | RCT | Humans | Laparoscopic Roux-en-Y gastric bypass | Insuflow device (Lexion Medical) | Heated (35°C), humidified (95% RH) CO2 | 25 | Cold, dry CO2 | 25 | No difference in analgesia requirement or abdominal pain; significant difference in shoulder pain at 18 hours (but not at 6, 12, 24, or 48 hours) | VAS |
| Yu et al. 2013 [26] | RCT | Humans | Laparoscopic appendectomy | Insuflow (MR 860, Fisher & Paykel Healthcare) | Heated (37°C), humidified (98% RH) CO2 | 97 | Cold (20-21°C), dry (0% RH) CO2 | 98 | No difference in quantity of analgesia required | (1) MEDD |
| No difference in pain on visual analogue score | (2) VAS | |||||||||
| Savel et al. 2005 [32] | RCT | Humans | Laparoscopic Roux-en-Y gastric bypass | Insuflow device (Lexion Medical) | Heated (35°C), humidified (95% RH) CO2 | 15 | Cold, dry CO2 | 15 | No difference in quantity of morphine required postoperatively | (1) Total morphine use |
| No difference in visual analogue score | (2) VAS | |||||||||
| Klugsberger et al. 2014 [27] | RCT | Humans | Laparoscopic cholecystectomy | Optitherm device ptorz, Tuttlingen, Germany) | Heated, humidified CO2 | 81 | Cold, dry CO2 | 67 | Lower visual analogue score in the heated, humidified CO2 group at postoperative day 0 | (1) Total analgesia requirement |
| No difference in total analgesia required | (2) VAS | |||||||||
| Herrmann and De Wilde 2015 [28] | RCT | Humans | Laparoscopic assisted vaginal hysterectomy | Insuflow (MR 860, Fisher & Paykel Healthcare) | Heated (37°C), humidified (98% RH) CO2 | 48 | Cold (20-21°C), dry (0% RH) CO2 | 49 | Lower total morphine consumption in warmed, humidified CO2 group (P = 0.02) | (1) Total morphine consumption |
| (2) VAS | ||||||||||
| Benavides et al. 2009 [41] | RCT | Humans | Laparoscopic gastric banding | Insuflow device (Lexion Medical) | Heated (35°C), humidified (95% RH) CO2 | 38 | Cold, dry CO2 | 35 | Significantly less postoperative pain in warmed, humidified CO2 group than in the cold, dry CO2 and heated, dry CO2 groups (P < 0.01, P < 0.05) | MEDD |
| Heated, dry CO2 | 40 | |||||||||
Table 4.
Effects of warmed, humidified carbon dioxide on respiratory function
| Study | Study type | Humans/animals | Operation | Device used |
Study group |
Control group |
Results | ||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | No. | Treatment | No. | ||||||
| Bashirov et al. 2007 [43] | Prospective, non-RCT | Animals (pigs) | Pneumoperitoneum creation | Model Ref L-70 NI Hotline (Sims-Smith Industries Medical Systems, Rockland, MA, USA) | Warmed CO2 groups | 6 (7°C), 6 (22°C), 6 (37°C) | No CO2 pneumoperitoneum | 6 | Increase in temperature of CO2 resulted in increased peritoneal CO2 absorption, increased PaCO2 and a greater decrease in pH (7.44 vs. 7.26) |
| Uzunkoy et al. 2006 [44] | RCT | Humans | Elective lap cholecystectomy | H-500 fluid warmer (Level 1 Technologies, Inc.,Rockland, MA, USA) | Warmed CO2 (37°C) | 15 | Cold CO2 (21°C) | 15 | Pulmonary function test performed 12 hours after the operation found lung function was significantly better in those receiving warmed CO2 (FVC, FEV1, PEF) |
| BergstrĂśm et al. 2008 [42] | Prospective, non-RCT | Animals (pigs) | Pneumoperitoneum creation | Laparoscopic insufflator (Storz, Tuttlingen, Germany) | CO2 | 10 | Helium | 10 | CO2 pneumoperitoneum resulted in significantly lower peritoneal pH (6.4 vs. 7.5, P = 0.001) |
| However, very minimal changes in arterial pH (7.43 vs. 7.49, P = 0.004) were found, with no clinical significance | |||||||||
| Ozgonul et al. 2007 [24] | RCT | Humans | Elective laparoscopic cholecystectomy | H-500 fluid warmer (Level 1 Technologies, Inc., MA, USA) | Warmed (37°C) CO2 | 31 | Cold (21°C) CO2 | 31 | No significant difference in arterial pH, pCO2, or HCO3- |
REFERENCES
1. Persson M, van der Linden J. Intraoperative CO2 insufflation can decrease the risk of surgical site infection. Med Hypotheses 2008;71:8â13.


2. Binda MM, Koninckx PR. Prevention of adhesion formation in a laparoscopic mouse model should combine local treatment with peritoneal cavity conditioning. Hum Reprod 2009;24:1473â9.


3. Tsuchiya M, Sato EF, Inoue M, Asada A. Open abdominal surgery increases intraoperative oxidative stress: can it be prevented? Anesth Analg 2008;107:1946â52.


4. van der Linden J, Persson M. CO2 field flooding may also reduce oxidative stress in open surgery. Anesth Analg 2009;109:683;author reply 683-4.


5. Hsieh CS, Tain YL, Chen YC, Chang K, Jean YH, Huang LT. Carbon dioxide pneumoperitoneum induces anti-inflammatory response and hepatic oxidative stress in young rats with bacterial peritonitis. Pediatr Surg Int 2011;27:289â94.


6. Persson M, van der Linden J. Wound ventilation with carbon dioxide: a simple method to prevent direct airborne contamination during cardiac surgery? J Hosp Infect 2004;56:131â6.


7. Targarona EM, Rodriguez M, Camacho M, Balague C, Gich I, Vila L, et al. Immediate peritoneal response to bacterial contamination during laparoscopic surgery. Surg Endosc 2006;20:316â21.


8. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1â12.


9. Howick J, Chalmers I, Glasziou P, Greenhalgh T, Heneghan C, Liberati A, et al. Explanation of the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) levels of evidence (background document) [Internet]. Oxford (UK), Centre for Evidence-Based Medicine; c2013 [cited 2016 Sep 1]. Available from: http://www.cebm.net/index.aspx?o=5653
10. Brokelman WJ, Holmdahl L, Bergstrom M, Falk P, Klinkenbijl JH, Reijnen MM. Heating of carbon dioxide during insufflation alters the peritoneal fibrinolytic response to laparoscopic surgery: a clinical trial. Surg Endosc 2008;22:1232â6.


11. Sammour T, Kahokehr A, Hayes J, Hulme-Moir M, Hill AG. Warming and humidification of insufflation carbon dioxide in laparoscopic colonic surgery: a double-blinded randomized controlled trial. Ann Surg 2010;251:1024â33.


12. Ivarsson ML, Bergstrom M, Eriksson E, Risberg B, Holmdahl L. Tissue markers as predictors of postoperative adhesions. Br J Surg 1998;85:1549â54.


13. Peng Y, Zheng M, Ye Q, Chen X, Yu B, Liu B. Heated and humidified CO2 prevents hypothermia, peritoneal injury, and intra-abdominal adhesions during prolonged laparoscopic insufflations. J Surg Res 2009;151:40â7.


14. Sammour T, Mittal A, Delahunt B, Phillips AR, Hill AG. Warming and humidification have no effect on oxidative stress during pneumoperitoneum in rats. Minim Invasive Ther Allied Technol 2011;20:329â37.


15. Hazebroek EJ, Schreve MA, Visser P, De Bruin RW, Marquet RL, Bonjer HJ. Impact of temperature and humidity of carbon dioxide pneumoperitoneum on body temperature and peritoneal morphology. J Laparoendosc Adv Surg Tech A 2002;12:355â64.


16. Margulis V, Matsumoto ED, Tunc L, Taylor G, Duchenne D, Cadeddu JA. Effect of warmed, humidified insufflation gas and anti-inflammatory agents on cytokine response to laparoscopic nephrectomy: porcine model. J Urol 2005;174(4 Pt 1): 1452â6.


17. Erikoglu M, Yol S, Avunduk MC, Erdemli E, Can A. Electron-microscopic alterations of the peritoneum after both cold and heated carbon dioxide pneumoperitoneum. J Surg Res 2005;125:73â7.


18. Moehrlen U, Ziegler U, Boneberg E, Reichmann E, Gitzelmann CA, Meuli M, et al. Impact of carbon dioxide versus air pneumoperitoneum on peritoneal cell migration and cell fate. Surg Endosc 2006;20:1607â13.


19. Ott DE. Correction of laparoscopic insufflation hypothermia. J Laparoendosc Surg 1991;1:183â6.


20. Frank SM, Beattie C, Christopherson R, Norris EJ, Perler BA, Williams GM, et al. Unintentional hypothermia is associated with postoperative myocardial ischemia. The Perioperative Ischemia Randomized Anesthesia Trial Study Group. Anesthesiology 1993;78:468â76.


21. Schmied H, Kurz A, Sessler DI, Kozek S, Reiter A. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet 1996;347:289â92.


22. Davis SS, Mikami DJ, Newlin M, Needleman BJ, Barrett MS, Fries R, et al. Heating and humidifying of carbon dioxide during pneumoperitoneum is not indicated: a prospective randomized trial. Surg Endosc 2006;20:153â8.


23. Farley DR, Greenlee SM, Larson DR, Harrington JR. Double-blind, prospective, randomized study of warmed, humidified carbon dioxide insufflation vs standard carbon dioxide for patients undergoing laparoscopic cholecystectomy. Arch Surg 2004;139:739â43. ;discussion 743-4.


24. Ozgonul A, Erkan C, Mehmet G, Zeynep B, Ali U. The effects of isothermic or hypothermic carbon dioxide pneumoperitoneum on arterial blood gases. Saudi Med J 2007;28:1662â5.

25. Manwaring JM, Readman E, Maher PJ. The effect of heated humidified carbon dioxide on postoperative pain, core temperature, and recovery times in patients having laparoscopic surgery: a randomized controlled trial. J Minim Invasive Gynecol 2008;15:161â5.


26. Yu TC, Hamill JK, Liley A, Hill AG. Warm, humidified carbon dioxide gas insufflation for laparoscopic appendicectomy in children: a double-blinded randomized controlled trial. Ann Surg 2013;257:44â53.


27. Klugsberger B, Schreiner M, Rothe A, Haas D, Oppelt P, Shamiyeh A. Warmed, humidified carbon dioxide insufflation versus standard carbon dioxide in laparoscopic cholecystectomy: a double-blinded randomized controlled trial. Surg Endosc 2014;28:2656â60.


28. Herrmann A, De Wilde RL. Insufflation with humidified and heated carbon dioxide in short-term laparoscopy: a double-blinded randomized controlled trial. Biomed Res Int 2015;2015:412618.




29. Hamza MA, Schneider BE, White PF, Recart A, Villegas L, Ogunnaike B, et al. Heated and humidified insufflation during laparoscopic gastric bypass surgery: effect on temperature, postoperative pain, and recovery outcomes. J Laparoendosc Adv Surg Tech A 2005;15:6â12.


30. Nguyen NT, Furdui G, Fleming NW, Lee SJ, Goldman CD, Singh A, et al. Effect of heated and humidified carbon dioxide gas on core temperature and postoperative pain: a randomized trial. Surg Endosc 2002;16:1050â4.



31. Bäcklund M, Kellokumpu I, Scheinin T, von Smitten K, Tikkanen I, Lindgren L. Effect of temperature of insufflated CO2 during and after prolonged laparoscopic surgery. Surg Endosc 1998;12:1126â30.


32. Savel RH, Balasubramanya S, Lasheen S, Gaprindashvili T, Arabov E, Fazylov RM, et al. Beneficial effects of humidified, warmed carbon dioxide insufflation during laparoscopic bariatric surgery: a randomized clinical trial. Obes Surg 2005;15:64â9.


33. Mouton WG, Bessell JR, Millard SH, Baxter PS, Maddern GJ. A randomized controlled trial assessing the benefit of humidified insufflation gas during laparoscopic surgery. Surg Endosc 1999;13:106â8.


34. Saad S, Minor I, Mohri T, Nagelschmidt M. The clinical impact of warmed insufflation carbon dioxide gas for laparoscopic cholecystectomy. Surg Endosc 2000;14:787â90.


35. Yeh CH, Kwok SY, Chan MK, Tjandra JJ. Prospective, case-matched study of heated and humidified carbon dioxide insufflation in laparoscopic colorectal surgery. Colorectal Dis 2007;9:695â700.


36. Champion JK, Williams M. Prospective randomized trial of heated humidified versus cold dry carbon dioxide insufflation during laparoscopic gastric bypass. Surg Obes Relat Dis 2006;2:445â9. ; discussion 449-50.


37. Nelskylä K, Yli-Hankala A, SjĂśberg J, Korhonen I, Korttila K. Warming of insufflation gas during laparoscopic hysterectomy: effect on body temperature and the autonomic nervous system. Acta Anaesthesiol Scand 1999;43:974â8.


38. Bessell JR, Karatassas A, Patterson JR, Jamieson GG, Maddern GJ. Hypothermia induced by laparoscopic insufflation. A randomized study in a pig model. Surg Endosc 1995;9:791â6.



39. Beste TM, Daucher JA, Holbert D. Humidified compared with dry, heated carbon dioxide at laparoscopy to reduce pain. Obstet Gynecol 2006;107(2 Pt 1): 263â8.


40. Kissler S, Haas M, Strohmeier R, Schmitt H, Rody A, Kaufmann M, et al. Effect of humidified and heated CO2 during gynecologic laparoscopic surgery on analgesic requirements and postoperative pain. J Am Assoc Gynecol Laparosc 2004;11:473â7.


41. Benavides R, Wong A, Nguyen H. Improved outcomes for lap-banding using the Insuflow device compared with heated-only gas. JSLS 2009;13:302â5.


42. BergstrĂśm M, Falk P, Park PO, Holmdahl L. Peritoneal and systemic pH during pneumoperitoneum with CO2 and helium in a pig model. Surg Endosc 2008;22:359â64.


43. Bashirov E, Cetiner S, Emre M, Seydaliyeva T, Alic V, Daglioglu K, et al. A randomized controlled study evaluating the effects of the temperature of insufflated CO2 on core body temperature and blood gases (an experimental study). Surg Endosc 2007;21:1820â5.


44. Uzunkoy A, Ozgonul A, Ceylan E, Gencer M. The effects of isothermic and hypothermic carbon dioxide pneumoperitoneum on respiratory function test results. J Hepatobiliary Pancreat Surg 2006;13:567â70.


45. Nduka CC, Puttick M, Coates P, Yong L, Peck D, Darzi A. Intraperitoneal hypothermia during surgery enhances postoperative tumor growth. Surg Endosc 2002;16:611â5.


46. Texler ML, King G, Hewett PJ. Tumour cell movement during heating and humidification of insufflating CO2: an in vitro model. Aust N Z J Surg 1998;68:740â2.


47. Tan BJ. Is carbon dioxide insufflation safe for laparoscopic surgery? A model to assess the effects of carbon dioxide on transitional-cell carcinoma growth, apoptosis, and necrosis. J Endourol 2006;20:965â9.


48. Marshall JK, Lindner P, Tait N, Maddocks T, Riepsamen A, van der Linden J. Intra-operative tissue oxygen tension is increased by local insufflation of humidified-warm CO2 during open abdominal surgery in a rat model. PLoS One 2015;10:e0122838.



49. Enfors SO, Molin G, TernstrĂśm A. Effect of packaging under carbon dioxide, nitrogen or air on the microbial flora of pork stored at 4 degrees C. J Appl Bacteriol 1979;47:197â208.


50. Enfors SO, Molin G. The influence of high concentrations of carbon dioxide on the germination of bacterial spores. J Appl Bacteriol 1978;45:279â85.


51. Molin G. The resistance to carbon dioxide of some food related bacteria. Eur J Appl Microbiol Biotechnol 1983;18:214â7.

52. Persson M, Svenarud P, Flock JI, van der Linden J. Carbon dioxide inhibits the growth rate of Staphylococcus aureus at body temperature. Surg Endosc 2005;19:91â4.


53. Taylor GJ, Bannister GC. Infection and interposition between ultraclean air source and wound. J Bone Joint Surg Br 1993;75:503â4.


54. Whyte W, Hodgson R, Tinkler J. The importance of airborne bacterial contamination of wounds. J Hosp Infect 1982;3:123â35.


55. Hanly EJ, Fuentes JM, Aurora AR, Bachman SL, De Maio A, Marohn MR, et al. Carbon dioxide pneumoperitoneum prevents mortality from sepsis. Surg Endosc 2006;20:1482â7.