- Search
| Ann Coloproctol > Volume 39(5); 2023 > Article |
|
Abstract
Purpose
A patient presented to a regional surgical center with Fournier gangrene (FG) and concurrent multifocal necrotizing fasciitis (NF). Given the rarity, it was decided to undertake a systematic review to investigate the incidence and prevalence of FG with multifocal NF and consequently determine the treatment and approach to management of such presentation.
Methods
Firstly, the report of the 56-year-old male patient is discussed regarding his surgical management. Secondly, a systematic review was undertaken according to PRISMA guidelines using MEDLINE, Scopus, and Embase databases. Searches used the following MeSH terms: (ŌĆ£fournierŌĆÖs gangreneŌĆØ) AND ((necrotising fasciitis) OR (necrotising soft tissue infection)). Once the search results were obtained, duplicate articles were removed. Titles, abstracts, and articles were reviewed by 2 authors.
Results
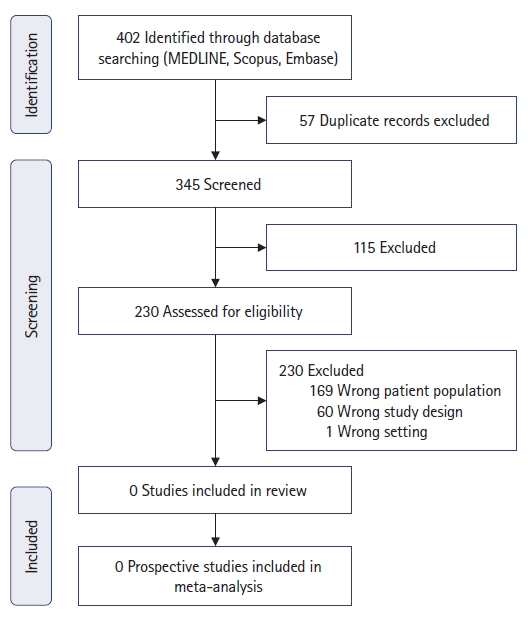
The search strategy using the 3 databases revealed a total of 402 studies. Fifty-seven studies were removed due to duplication. A total of 345 records were screened via title and abstract, of which 115 were excluded. Two hundred and thirty studies were reviewed for eligibility. A total of all 230 studies were excluded; 169 were excluded as they included the incorrect patient population (patients suffered from FG or NF, but not both collectively), 60 studies were excluded due to incorrect study designs, and 1 report occurred in the wrong setting.
Necrotizing fasciitis (NF) or necrotizing soft tissue infection is a rapidly progressive infection of skin, fat, fascia, and muscle, typically involving the limbs and groin [1]. Fournier gangrene (FG) is a form of NF affecting the perineum, perianal and genital regions [2]. Both are life-threatening infections that involve rapid necrosis of bodily tissue. Mortality rates of NF and FG have been reported at 21.1% [3] and 12.5% [4], respectively, in recent meta-analyses.
While dozens of reports have documented multifocal NF [5], to our knowledge, there are no reports of FG with concurrent multifocal NF in the literature. Multifocal NF is defined as 2 or more areas of noncontiguous and noncommunicating areas of necrosis [5]. The primary risk factors identified for multifocal NF included male sex, lower limb involvement, and marine gram-negative bacterial organisms [6]. Patients with multifocal NF have been deemed to have a high mortality rate (62% vs. 30%) compared with a single-site infection [6]. Two theories currently exist for the development of multifocal NF: either the development of septic embolization metastatically or concurrent multifocal inoculation and thus development of further NF [7]. The primary risk factors identified for FG include diabetes, alcoholism, poor nutritional state, malignancies, and recent trauma to the perineum [8].
Firstly, we present a case of scrotal FG with multifocal NF of the extremities. Secondly, we conduct a systematic review of the literature to determine if similar cases have been reported in the past.
Ethical approval was obtained from the local health services Human Ethics Review Committee-Institutional Review Body (No. LNR/65474/BHCG-2020-217550 [v1]) on June 1, 2021. Written informed signed consent was obtained from the patient for his anonymized information to be published in this article.
This review was conducted according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. An electronic literature review was conducted using MEDLINE, Scopus, and Embase databases for publications between 1980 and 2021 in the English language. Initial searches used the following MeSH (Medical Subject Headings) terms: (ŌĆ£fournierŌĆÖs gangreneŌĆØ) AND ((necrotising fasciitis) OR (necrotising soft tissue infection)). Once the search results were obtained, duplicate articles were removed. If authors/groups with multiple papers studying the same patient cohort were identified, we included only the most recent study to avoid duplication. Titles, abstracts, and full articles were reviewed by 2 authors (JAP and CHAL). This was used to determine that articles addressed inclusion criteria. To assess eligibility for the systematic review, PICO (population, intervention, comparison, outcome) criteria were used. The PICO criteria were as follows: population, case reports or case series of FG and NF; intervention, none; comparison, none; and outcome, clinical presentations, treatment strategies, and survival.
A 56-year-old male patient presented to a rural Victorian Urgent Care Center with 4 days of scrotal pain, myalgia, and inability to walk. His medical history consisted of alcohol abuse and 40 pack-year smoking history. He worked as a fruit picker and had previously been a pig farmer. Examination revealed fulminant necrosis of the scrotum, in addition to the dorsum and heels of both feet, and the left middle finger. He was commenced on intravenous benzylpenicillin and flucloxacillin and transferred to the nearest regional center. On arrival at the regional center, his examination findings were consistent were concerning multifocal NF with FG as shown in Fig. 1. A diagnosis of scrotal FG with concurrent multifocal NF was made at the regional center.
On arrival at the regional center, antibiotic therapy was changed to intravenous clindamycin, meropenem, and vancomycin. He was urgently transferred to the operating room for surgical exploration and debridement. Intraoperative findings included global necrosis of scrotum associated with copious purulent discharge and bilateral nonviable testes. He underwent scrotal debridement, bilateral orchiectomy, and debridement of necrotic tissue from both feet and left middle finger. Postoperatively, he was admitted to the intensive care unit (ICU) of the regional center for 1 day before being transferred to a metropolitan tertiary institution. The patient remained intubated during this time while receiving intravenous vasopressor support (noradrenaline) and antibiotics. Pathological analysis of scrotal tissue was consistent with FG and tissue culture revealed polymicrobial growth including Streptococcus intermedius, Escherichia coli, Klebsiella oxytoca, and Staphylococcus aureus. Histological examination of limb tissue was consistent with NF.
At the metropolitan center, the patient spent a further 6 days in ICU (and a further 4 days on noradrenaline vasopressor support), 24 days on the surgical ward, and underwent 3 further operations. On day 2, he underwent scrotal debridement, left medial thigh exploration, and insertion of bilateral middle ear ventilation tubes (to prevent middle ear barotrauma) with general surgery, plastic surgery, urology, and otorhinolaryngology teams. On day 8, he underwent scrotal wound reexploration and closure, and further debridement of limb wounds with urology and plastic surgery. On day 16, he underwent further debridement and received split skin grafts from the left thigh to bilateral heel wounds. It is not clinically perceived that the use of vasopressors contributed to the development of the multifocal NF; however, the ongoing use of vasopressors may have delayed the skinŌĆÖs inflammatory and healing response.
The patient was discharged on day 30 of his tertiary admission to a regional rehabilitation facility. His scrotal wound had stabilized and bilateral skin grafts showed good uptake. He was downgraded to oral ciprofloxacin and amoxicillin/clavulanate on discharge. Following bilateral orchiectomy, he was commenced on lifelong testosterone replacement therapy. He was discharged home after 60 days of inpatient rehabilitation after making a full recovery. The focus of FG was believed to be genitourinary, given the extent of scrotal involvement with necrotic testes, the multifocal involvement, and the patientŌĆÖs otherwise good preexisting health.
He is well 5 months on from his initial presentation and plans to return to fruit picking in the summer.
The search strategy using the 3 databases revealed a total of 402 studies. Fifty-seven studies were removed prior to screening due to duplication. A total of 345 records were screened via title and abstract, of which 115 were excluded. As shown in Fig. 2, 230 studies were reviewed for eligibility. A total of 230 studies were excluded: 169 were excluded as they included the incorrect patient population (patients suffered from FG or NF, but not both collectively); 60 studies were not included as they were the incorrect study designs; and 1 report occurred in the wrong setting. Of the 230 studies excluded, 127 studies purely discussed patientŌĆÖs suffering from FG without multifocal NF. A further 42 studies purely discussed patientŌĆÖs suffering from NF not involving the perineum (FG). Of these 169 studies reviewed, none reported any patients diagnosed with FG and multifocal NF. Furthermore, of these 169 studies, 62 were case reports, 104 were case series, and 3 were cohort studies. In total, 61 full studies were irrelevant as detailed in the PRISMA flowchart below in Fig. 2. One study [9] which was performed within the wrong setting included a report assessing the biomarkers as the association of NF severity and the incidence of FG with second-line antidiabetic drugs.
This systematic review reviewed 230 studies for full review. Of these 230 studies, none reported FG with multifocal NF. All case reports, case series, or cohort studies discussed FG or NF as separate entities while not presenting simultaneously with each other. This highlights the rarity of such combined presentations.
Multifocal NF, while rare, has been reported in the literature. El-khani et al. [5] performed a systematic review of multifocal NF with 33 individual cases over 50 years. They hypothesized that multifocal NF appears to be underestimated and underreported due to the lack of local registries and it is omission as a reportable disease in many nations. Therefore, the true incidence of multifocal NF is not unknown. Furthermore, their review highlighted time delays between the appearances of multifocal NF lesions impacting diagnosis of the condition. Individual lesions can occur at different times, acute and subacute, often with extended time delays [5]. For example, Toledo et al. [10] report a case of multifocal NF developing 48 hours after the initial lesion was identified. A pediatric patient initially developed right lower extremity NF, followed 48 hours later by the left upper extremity developing NF. Lee et al. [6] also reported multifocal NF 48 and 120 hours after initial presentation. Three additional articles also highlighted this time delay [11ŌĆō13] emphasizing the importance of frequent reexamination of patients with FG or NF to ensure early detection of multifocal involvement.
El-khani et al. [5] hypothesized that delayed presentation of secondary lesions may occur from metastatic septic embolization or hematologous spread. This was suggested from the theory that NF commonly affects the abdominal wall, extremities, and perineum; however, the limited multifocal NF reported commonly occurs in the extremities, suggesting a systemic spread, rather than local expansion. This was further supported by Herrod et al. [14], who reported a case presentation of multifocal NF; they hypothesized that instead of the classic NF spreading by direct invasion along fascial planes, it is disseminated by septic emboli in systemic circulation. The 81-year-old patient developed both right thigh and left forearm NF following the diagnosis of ischemic colitis.
Several risk factors for the development of multifocal NF have been described including male sex, lower limb involvement, liver cirrhosis, end-stage renal disease, and marine gram-negative bacterial infection [6]. Noncontiguous multifocal NF has been attributed to a high frequency of gram-negative marine bacteria [15]. Both Park et al. [15] and Lee et al. [6] reported an overall higher mortality rate of multifocal NF compared to monofocal NF. Park et al. [15] reported a mortality of 62%, and Lee et al. [6] reported 67%, which is higher than the cumulative average mortality rate of 34% reported for monofocal NF within the literature. This has been further identified by Hua et al. [16] who stated that multifocal NF was an independent risk factor for NF mortality.
The presence of multifocal NF reports within the literature, but no documentation of concurrent FG emphasizes the rarity and seriousness of the presentation experienced within this case report. Furthermore, it could be explained that authors within the literature may not provide sufficient case detail on multifocality when reporting on large case series. It is very likely that the true number of multifocal NF, and multifocal NF with concurrent FG is much higher in the clinical setting than reported within the literature.
Novel risk factors have been reported in the literature, including sodium-glucose cotransporter-2 (SGLT2) inhibitors and immunosuppression [16ŌĆō23]. SGLT2 inhibitors are a recent introduction to oral hypoglycemic agents. They decrease glucose reabsorption and the promotion of glucosuria which consequently reduces plasma glucose independently of insulin. It is hypothesized that SLGT2 inhibitors can potentiate the risk of urogenital tract and genital infections, and therefore predispose to greater surrounding infections of the perineum [17]. Immunosuppression is a theme reflected by Creta et al. [2] determining that patients suffering from hematological malignancies are at increased risk of developing FG. Patients suffering from malignancies have multiple hospital encounters and admissions; therefore, they are at higher risk of exposure to nosocomial, multidrug-resistant organisms which are commonly encountered in FG. It should also be highlighted that NF can often represent the first clinical indication of malignancy [18ŌĆō20].
Surgical scrotal reconstruction techniques post-FG also feature predominantly in the literature [19, 21ŌĆō26]. The loss of skin and the subsequent layers of perineal tissue following necrotizing infection poses a reconstructive challenge. The challenges that required large tissue coverage, combined with the prior need for radical debridement and intensive care support for the patient, create a difficult surgical situation. This emphasizes the extensive multidisciplinary team required for treating multifocal NF and FG, who all need to be cognitive of the need for reexamination of the patient to ensure no further lesions have developed.
An additional consideration in our case was the use of vasopressors and inotropes in the intensive care setting. NF or FG patients are often hemodynamically unstable and require pharmacological support for blood pressure and cardiac output. There is, however, evidence that the use of vasopressors or inotropes is a predictive risk factor for mortality of NF or FG patients in the ICU as discussed by Peetermans et al. [27]; however, it does not seem to worsen NF or FG.
This systematic review highlights that while multifocal NF has been reported throughout the literature, nil documentation of FG with multifocal NF has been documented within the literature. While it is anticipated that this has occurred within the clinical setting, due to the lack of documentation within the literature this cannot be proven.
This systematic review highlights that while being a relatively known, uncommon infection both NF and FG are well documented separately within the literature. However, no reports of FG with concurrent multifocal NF have been documented within the literature. Despite its rare presentation, this highlights the need for comprehensive and frequent reexamination of such patients to monitor for the development of multifocal NF with FG or multifocal NF.
REFERENCES
2. Creta M, Sica A, Napolitano L, Celentano G, La Rocca R, Capece M, et al. FournierŌĆÖs gangrene in patients with oncohematological diseases: a systematic review of published cases. Healthcare (Basel) 2021;9:1123.



3. Nawijn F, Smeeing DP, Houwert RM, Leenen LP, Hietbrink F. Time is of the essence when treating necrotizing soft tissue infections: a systematic review and meta-analysis. World J Emerg Surg 2020;15:4.




4. El-Qushayri AE, Khalaf KM, Dahy A, Mahmoud AR, Benmelouka AY, Ghozy S, et al. FournierŌĆÖs gangrene mortality: a 17-year systematic review and meta-analysis. Int J Infect Dis 2020;92:218ŌĆō25.


5. El-khani U, Nehme J, Darwish A, Jamnadas-Khoda B, Scerri G, Heppell S, et al. Multifocal necrotising fasciitis: an overlooked entity? J Plast Reconstr Aesthet Surg 2012;65:501ŌĆō12.


6. Lee CY, Li YY, Huang TW, Huang TY, Hsu WH, Tsai YH, et al. Synchronous multifocal necrotizing fasciitis prognostic factors: a retrospective case series study in a single center. Infection 2016;44:757ŌĆō63.




7. Espandar R, Sibdari SY, Rafiee E, Yazdanian S. Necrotizing fasciitis of the extremities: a prospective study. Strategies Trauma Limb Reconstr 2011;6:121ŌĆō5.




8. Ten├│rio CE, Lima SV, Albuquerque AV, Cavalcanti MP, Teles F. Risk factors for mortality in FournierŌĆÖs gangrene in a general hospital: use of simplified Founier gangrene severe index score (SFGSI). Int Braz J Urol 2018;44:95ŌĆō101.



9. Hedetoft M, Hansen MB, Madsen MB, Johansen JS, Hyldegaard O. Associations between YKL-40 and markers of disease severity and death in patients with necrotizing soft-tissue infection. BMC Infect Dis 2021;21:1046.




10. Toledo JD, L├│pez-Prats JL, Ibiza E, Modesto V, Sanchis R, Vento M. Case 2: an 18-month-old child with necrotic lesions on the limbs. Acta Paediatr 2006;95:1506ŌĆō8.


11. Liu SY, Ng SS, Lee JF. Multi-limb necrotizing fasciitis in a patient with rectal cancer. World J Gastroenterol 2006;12:5256ŌĆō8.



12. Rai RK, Londhe S, Sinha S, Campbell AC, Aburiziq IS. Spontaneous bifocal Clostridium septicum gas gangrene. J Bone Joint Surg Br 2001;83:115ŌĆō6.



13. Gardam MA, Low DE, Saginur R, Miller MA. Group B streptococcal necrotizing fasciitis and streptococcal toxic shock-like syndrome in adults. Arch Intern Med 1998;158:1704ŌĆō8.


14. Herrod PJ, Boghossian S, Vasas P. Multifocal necrotising fasciitis: a rarer presentation of a rare disease. BMJ Case Rep 2014;2014:bcr2014207089.



15. Park KH, Jung SI, Jung YS, Shin JH, Hwang JH. Marine bacteria as a leading cause of necrotizing fasciitis in coastal areas of South Korea. Am J Trop Med Hyg 2009;80:646ŌĆō50.


16. Hua C, Sbidian E, Hemery F, Decousser JW, Bosc R, Amathieu R, et al. Prognostic factors in necrotizing soft-tissue infections (NSTI): a cohort study. J Am Acad Dermatol 2015;73:1006ŌĆō12.


17. Hu Y, Bai Z, Tang Y, Liu R, Zhao B, Gong J, et al. Fournier gangrene associated with sodium-glucose cotransporter-2 inhibitors: a pharmacovigilance study with data from the U.S. FDA adverse event reporting system. J Diabetes Res 2020;2020:3695101.




18. Matsumura N, Nakamura Y, Hara I. Male urethral cancer associated with FournierŌĆÖs gangrene. Int J Urol 2009;16:589.


19. Schmitz M, Ludolph I, Horch RE. Staged reconstruction of challenging abdominal full thickness wounds caused by necrotizing fasciitis and complicated by occult rectal cancer: a rare combination. Int J Colorectal Dis 2016;31:769ŌĆō70.



21. Han HH, Lee JH, Kim SM, Jun YJ, Kim YJ. Scrotal reconstruction using a superficial circumflex iliac artery perforator flap following FournierŌĆÖs gangrene. Int Wound J 2016;13:996ŌĆō9.


22. Onyekwelu O, Reid A, McGrouther DA. Reconstruction of perineo-scrotal defects from FournierŌĆÖs gangrene with the adipofascial anterolateral thigh flap. J Clin Urol 2015;8:33ŌĆō7.


23. Dent BL, Dinesh A, Khan K, Engdahl R. Scrotal reconstruction with integra following necrotizing fasciitis. J Emerg Trauma Shock 2018;11:57ŌĆō9.



24. Chen SY, Fu JP, Chen TM, Chen SG. Reconstruction of scrotal and perineal defects in FournierŌĆÖs gangrene. J Plast Reconstr Aesthet Surg 2011;64:528ŌĆō34.


25. Ryssel H, Germann G, Czermak C, Kloeters O, Gazyakan E, Riedel K. Matriderm┬« in depth-adjusted reconstruction of necrotising fasciitis defects. Burns 2010;36:1107ŌĆō11.


26. Guzzetti T, Ferrario A. A reconstructive approach and an unusual dressing for genital skin loss after FournierŌĆÖs gangrene. Chirurgia 2003;16:127ŌĆō30.
- TOOLS