- Search
| Ann Coloproctol > Epub ahead of print |
|
Abstract
Purpose
We compared the incidence of venous thromboembolism (VTE) among Asian populations with localized colorectal cancer undergoing curative resection with and without the use of pharmacological thromboprophylaxis (PTP).
Methods
A comprehensive literature search was undertaken to identify relevant studies published from January 1, 1980 to February 28, 2022. The inclusion criteria were patients who underwent primary tumor resection for localized nonmetastatic colorectal cancer; an Asian population or studies conducted in an Asian country; randomized controlled trials, case-control studies, or cohort studies; and the incidence of symptomatic VTE, deep vein thrombosis, and/or pulmonary embolism as the primary study outcomes. Data were pooled using a random-effects model. This study was registered in PROSPERO on October 11, 2020 (No. CRD42020206793).
Results
Seven studies (2 randomized controlled trials and 5 observational cohort studies) were included, encompassing 5,302 patients. The overall incidence of VTE was 1.4%. The use of PTP did not significantly reduce overall VTE incidence: 1.1% (95% confidence interval [CI], 0%–3.1%) versus 1.9% (95% CI, 0.3%–4.4%; P=0.55). Similarly, PTP was not associated with significantly lower rates of symptomatic VTE, proximal deep vein thrombosis, or pulmonary embolism.
Conclusion
The benefit of PTP in reducing VTE incidence among Asian patients undergoing curative resection for localized colorectal cancer has not been clearly established. The decision to administer PTP should be evaluated on a case-by-case basis and with consideration of associated bleeding risks.
Venous thromboembolism (VTE), which includes deep venous thrombosis (DVT) and pulmonary embolism (PE), is a common and potentially preventable condition in hospitalized and surgical patients. VTE is associated with a significant risk of morbidity and mortality if left untreated. After lung cancer, colorectal cancer (CRC) is the second most common type of malignancy, and CRC patients are susceptible to developing VTE [1]. The use of mechanical thromboprophylaxis (MTP) modalities, such as intermittent pneumatic compression (IPC) or graduated compression stockings (GCS), and pharmacological thromboprophylaxis (PTP) using agents such as enoxaparin and fondaparinux can potentially reduce the risk of VTE in CRC patients [2, 3]. However, the decision to administer PTP is complex, as it depends upon the perceived risk of developing VTE, and must be carefully balanced with the potential for bleeding complications associated with PTP. The American Hematology Society has established a set of guidelines for administering thromboprophylaxis [4], and the American Society of Colon and Rectal Surgeons has published guidelines specifically for patients undergoing colorectal surgery [5].
Nevertheless, one caveat regarding the use of these guidelines is that they were developed from studies on mostly Western populations. However, it appears that VTE occurs less frequently in Asian populations than in Western populations [6, 7]. Even when environmental influences were accounted for, Hispanic ethnicity and Asian/Pacific race were independently associated with a lower risk of VTE than observed in Westerners [8]. In recent studies, the VTE incidence rate reported in Asian populations ranged from 14 to 57 per 100,000 [9–13]. In contrast, the incidence rate reported in Western populations was from 75 to 143 per 100,000 [14–16]. Therefore, given the lower reported incidence of VTE among Asian patients, routine PTP may result in unnecessary costs and increase the risk of bleeding complications.
At present, there is a paucity of high-quality literature pertaining to VTE incidence and prophylaxis in Asian CRC patients. Furthermore, no consensus has been established regarding the use of PTP in this population. In this study, we evaluated the evidence in the literature regarding the use of PTP in reducing the incidence of VTE in patients with localized CRC undergoing curative resection in the Asian context.
This review was carried out and reported in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for systematic reviews and meta-analyses. A protocol was developed a priori by the reviewers and was registered in the PROSPERO database on October 11, 2020 (No. CRD42020206793).
A structured and comprehensive electronic search from January 1, 1980 to February 28, 2022 was conducted systematically using PubMed, Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, and Google Scholar. We defined an Asian population as people living in the geographical regions of East Asia, South-East Asia, and South Asia. The keywords used were “Asia,” “Asian population,” “venous thromboembolism,” “deep vein thrombosis,” “pulmonary embolism,” “pharmacological thromboprophylaxis,” “mechanical thromboprophylaxis,” and “colorectal cancer surgery.” Additional articles that may have been overlooked in the initial search were identified by using the “related articles” feature in PubMed. Key references of the short-listed studies were also searched manually.
Two investigators (SJJT and YYRN) independently performed the search and assessed each article for eligibility. Discrepancies were resolved by discussion between SJJT and YYRN to reach a consensus or in consultation with a third reviewer (AYC). Studies included on the final shortlist were decided by consensus among all 3 co-authors.
The study inclusion criteria were patients who underwent primary tumor resection for localized nonmetastatic CRC; studies with primarily Asian populations or that were conducted in an Asian country; randomized controlled trials (RCTs), case-control studies, or cohort studies; and the incidence of DVT, PE, and/or symptomatic VTE as the primary study outcomes. In addition, the following exclusion criteria were applied: patients with metastatic CRC; studies written in languages other than English; systematic reviews, commentaries, and editorials; and studies employing noncontemporary methods for diagnosing DVT or PE (e.g., ascending venogram, I-labelled fibrinogen test).
The following data were extracted from the included studies: first author; year of publication; country; number of patients; methods of thromboprophylaxis; the incidence of VTE, symptomatic VTE, proximal and distal DVT, PE, and bleeding complications in the form of major bleeding and minor bleeding where applicable; observation period for the primary outcome; and laparoscopic versus open resection.
The primary outcome was the incidence of VTE (calculated as the total number of DVT and PE events), symptomatic VTE, proximal DVT, and PE. The impacts of using PTP on the incidence of VTE and bleeding complications were evaluated as secondary outcomes. Data collection for the incidence of VTE, symptomatic VTE, proximal and distal DVT, and PE was performed with patients divided into 2 groups: with PTP and without PTP. The latter group also included those who received MTP (GCS or IPC). In the case of 2-armed studies that compared events with and without PTP, each arm was included as a separate cohort in the analysis.
Symptomatic VTE was defined as having clinical manifestations such as swelling and pain of lower extremities for DVT and a combination of chest pain, dyspnea, tachypnea, or poor oxygen saturation for PE, with confirmation of diagnosis on imaging. DVT was classified as proximal if the thrombus was located in the iliac, femoral, and/or popliteal vein. Major bleeding was defined by the presence of 1 or more of the following: fatal bleeding; bleeding that was retroperitoneal, intracranial, intraspinal, or involving any other critical organ; bleeding leading to reoperation or intervention; and/or bleeding causing a decrease of hemoglobin level of 2 g/dL or more.
The modified Downs and Black assessment tool was used to assess the methodological quality of the included studies, as it allows evaluation of both randomized and nonrandomized comparative studies [17]. The checklist consists of 27 items that address the following methodological components: reporting, external validity, internal validity (bias and confounding), and power. Twenty-six items are rated either as yes (1) or no/unable to determine (0), and 1 item is rated on a 3-point scale (yes, 2; partial, 1; no, 0). Scores range from 0 to 28, with higher scores indicating a better methodological quality of study. The following cut-points have been suggested to categorize studies by quality: excellent (26–28), good (20–25), fair (15–19), and poor (< 14).
The primary outcome and all secondary outcomes were treated as binary data. An inverse-variance DerSimonian and Laird random-effects model was used to account for heterogeneity among studies for both the primary and secondary outcomes. All results were presented in forest plots. All outcomes were expressed as the pooled event rate with 95% confidence intervals (CIs).
The restricted maximum likelihood, random-effects meta-regression approach was used to compare VTE incidence between the with-PTP and without-PTP groups. The heterogeneity of the included studies was evaluated using the Cochran Q test and the I2 index. Additionally, publication bias was evaluated using funnel plots in conjunction with Egger regression and the Begg and Mazumdar rank test. All tests were 2-tailed, and statistical significance was set at P< 0.05. All statistical analyses were conducted using Comprehensive Meta-Analysis ver. 3 (Biostat).
The search strategy yielded a total of 37 articles that met the criteria for full-text review (Fig. 1). Three studies were excluded because they were published in Japanese and Korean. Twenty-seven additional articles were excluded because due to the use of noncontemporary methods of diagnosing DVT (ascending venogram, I-labelled fibrinogen), inappropriate or overlapping population groups, or inadequate data for extraction. After review, 7 articles were included [18–24]. The selection process flow diagram is shown in Fig. 1.
The details of the included studies are summarized in Table 1 [18–24]. Of the 7 included studies, encompassing 5,302 patients, 4 studies were from Japan, 2 from Korea, and 1 from India with the following patient numbers: 4,162 Koreans (78.5%), 1,041 Japanese (19.6%), and 99 Indians (1.9%). Two studies were RCTs and 5 were observational cohort studies. All studies used contemporary methods (e.g., Doppler venous ultrasonography, contrast venography, ascending phlebography, chest computed tomography [CT], or ventilation-perfusion scans) for diagnosing VTE.
The assessment protocols varied among the included studies. In 4 studies and cohort B in Lee et al. [19], further diagnostic imaging was performed only if there was clinical suspicion of DVT [18, 19, 23, 24] or if the D-dimer score was greater than 1 µg/mL on the 2nd and 7th postoperative days [22]. In the 2 remaining studies and cohort A in Lee et al. [19], all patients underwent routine diagnostic screening [20, 21]. For the assessment of PE, only symptomatic PE was investigated further in all studies.
The duration of VTE surveillance in most studies ranged between 10 to 30 days after receiving PTP, although in 1 study, the period of evaluation was up to 1 day after completion of PTP [18] and in another study, day 6±1 after commencement of PTP [21]. The commencement of PTP varied among the studies from 1 day prior to surgery to immediately after surgery. There was also heterogeneity in the choice of PTP agents (fondaparinux, enoxaparin, or dalteparin sodium) and dosage used among the studies. The majority of the studies allowed the use of MTP in the form of compression stockings or IPC, except 1 study where this was not specified [21]. Of the 7 studies, 4 studies further evaluated bleeding events [18, 20–22].
Methodological quality scores based on the modified Downs and Black checklist are presented in Table 1 [18–24]. With regards to study scoring, some studies did not satisfy certain criteria because the information was not available from the publication. According to the Downs and Black scoring criterion, if the study did not explicitly state a certain requested methodology for a particular item, that item must be scored as not satisfying the criterion. The mean±standard deviation for modified Downs and Black risk of bias checklist score was 21.0±0. The quality of the included studies was excellent (n=1), good (n=4), or fair (n=2). The methodological rating criteria most frequently satisfied in the papers reviewed were related to the representativeness of the sample group and adjustment for confounding factors in the data analysis.
The overall incidence of VTE among Asian CRC patients in the 7 included studies was 1.4% (95% CI, 0.4%–2.6%). The overall pooled incidence of symptomatic VTE, proximal DVT, and PE was 0.2% (95% CI, 0%–0.5%), 0.1% (95% CI, 0%–0.3%), and 0.1% (95% CI, 0%–0.3%), respectively.
Figs. 3–5 [18–24] show forest plots of the incidences of symptomatic VTE, proximal DVT, and PE, respectively, between the with-PTP and without-PTP groups. Comparing the 2 groups, the use of PTP was not shown to have statistical significance in reducing the incidence of symptomatic VTE (0.1% [95% CI, 0%–0.5%] vs. 0.4% [95% CI, 0.2%–0.8%], P=0.12), proximal DVT (0% [95% CI, 0%–0.2%] vs. 0.6% [95% CI, 0%–1.3%], P=0.09), and PE (0% [95% CI, 0%–0.2%] vs. 0.3% [95% CI, 0%–0.7%], P=0.30).
Four studies reported the incidence of bleeding complications with the use of PTP [18, 20–22]. Of the 4 studies, only 2 compared bleeding complications with and without PTP use [20, 21]. Among the patients who received PTP, the overall incidence of bleeding was 7.8%, of which the majority (7.0%) was minor in severity, compared to 0% reported in 2 studies among the patients who did not receive PTP.
The use of PTP in Asian surgical populations for CRC surgery is not a universal practice due to the lack of specific guidelines. The Asian Venous Thrombosis Forum working group has proposed general guidelines, but none were specific for CRC surgery [25]. Therefore, at present, the decision for VTE prophylaxis in Asian countries has been made pragmatically based on various factors including the individual patient risk profile, surgeon discretion, and institution protocol.
To the best of our knowledge, this is the first-ever systematic review and meta-analysis to comprehensively evaluate the incidence of VTE with and without PTP among Asian patients undergoing curative resection for localized CRC, including clinically relevant outcomes such as symptomatic VTE, proximal DVT, and PE. The included studies are all relatively recent, thereby more accurately reflecting contemporary operative management, perioperative thromboprophylaxis protocols, and prevailing obesity rates among Asian patients. The overall pooled incidence of VTE for our 7 studies was 1.4%, which was lower than has been reported in Western-based studies, where incidence rates of 2.4% to 17% have been reported [26–28]. A systematic review conducted among Asian patients undergoing orthopedic surgery also reported similar trends [29].
Our results suggest that the use of PTP does not significantly lower the risk of VTE events in CRC surgery patients in Asian populations, including overall VTE, symptomatic VTE, proximal DVT, and PE. Despite a trend towards reduced VTE incidence, the effect did not reach statistical significance. This is in contrast to results obtained from studies in Western populations [30, 31]. A meta-analysis on VTE prevention in general surgery based on studies obtained from Western populations reported a significant risk reduction of 70% in clinical VTE with the use of PTP [30]. Likewise, Turpie et al. [31] conducted an RCT in 1,309 patients undergoing abdominal surgery, comparing fondaparinux and a control group. The study reported a significant difference in the incidence of VTE when fondaparinux was given (1.7%) compared to the control group (5.3%). The discrepancy in findings between Asian and Western populations may be explained by postulations on differences in the environment, diet, postoperative hemostatic response [32, 33], and prothrombotic factors between Asian and Western populations [34, 35].
The risk of bleeding is considerable; therefore, it should be an important factor to take into consideration when introducing PTP in this population group. Of note, our overall incidence of bleeding events was 7.8%. Interestingly, this was significantly higher than a similar study by Moubayed et al. [36] conducted among patients undergoing otolaryngology-head and neck surgery, which reported an incidence of 0.9%. This result was consistent with another review on the influences of ethnic differences in prothrombotic and bleeding diatheses in patients undergoing microsurgical breast reconstruction. That study reported a significantly higher rate of bleeding events in Asian patients than in Western patients (2.6% vs. 1.2%, P=0.002) [37]. These findings further augment the emerging body of evidence suggesting possible differences in the coagulative responses between Asian and Western populations [33, 37]. As such, the introduction of PTP in this population group should be a calculated decision, one that takes into account the intrinsic differences in hemostatic pathways that result in a lower incidence of VTE events and conversely, higher bleeding risks.
The findings of our study must be considered in the context of its limitations. Firstly, there was heterogeneity among the included studies due in part to inconsistency in the definition of VTE, varying rates of minimally invasive versus open surgery, differences in tumor locations (colon vs. rectum), and disparities in mechanical and pharmacological thromboprophylaxis protocols among centers. Secondly, the 7 included studies originated from 3 Asian countries, consisting of predominantly Koreans, who accounted for a substantial percentage (78.5%); therefore, the findings may not be representative of all Asian populations. Thirdly, there was variation amongst the drugs and dosages used for PTP used, with differences among fondaparinux, enoxaparin, and dalteparin sodium. Furthermore, it has been previously reported that the median number of days from surgery to VTE occurrence was 10 [38]. The period of VTE surveillance ranged from 4 to 30 days postoperatively across the included studies, and shorter surveillance periods may have contributed to under-estimation of the actual VTE incidence, although the implications remain unclear given the low overall VTE incidence reported in this study.
This review highlights the role of PTP in VTE risk reduction for CRC surgery among Asian populations. This study shows a different picture among Asian populations compared to Western populations. Therefore, guidelines that were established based on Western populations should not be extrapolated for use in Asian populations. Although the available evidence is limited, study results would indicate discretion in the use of PTP for Asian populations due to the comparatively low beneficial yield for VTE risk reduction. Although other types of cancer surgery were not within the scope of this study, it is not unreasonable to consider that similar results may be identified for other cancer groups among Asian populations. It is worth noting that 60% (around 4.5 billion) of the world’s population is in Asia [39], and even in Western countries, Asian populations make up a substantial proportion of the population. It is high time that this research gap is rectified. Hence, it is of crucial importance that further large-scale, high-quality RCTs, including various Asian subpopulations, are carried out with the objective of formulating an optimal strategy and guidelines for the prophylactic management of VTE in Asian cancer surgery patients.
In conclusion, our review confirmed that the incidence of VTE among Asian populations is relatively low. The use of PTP was not shown to significantly reduce VTE risk. Therefore, the decision to introduce PTP must be evaluated on a case-by-case basis. Future high quality, comprehensive studies are required to acquire greater knowledge on the use of PTP in Asia.
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram showing the article search.
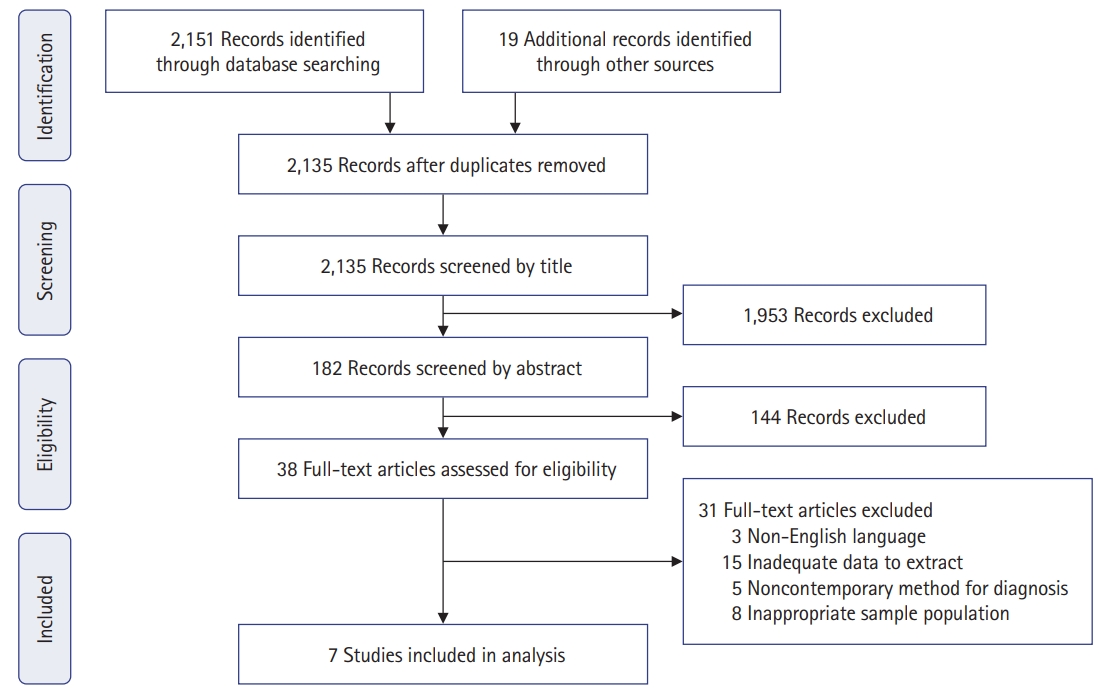
Fig. 2.
Forest plot and funnel plot comparing the overall incidence of venous thromboembolism (VTE) between pharmacological thromboprophylaxis and no pharmacological thromboprophylaxis. IV, interval variable; Random, random-effects model; CI, confidence interval.
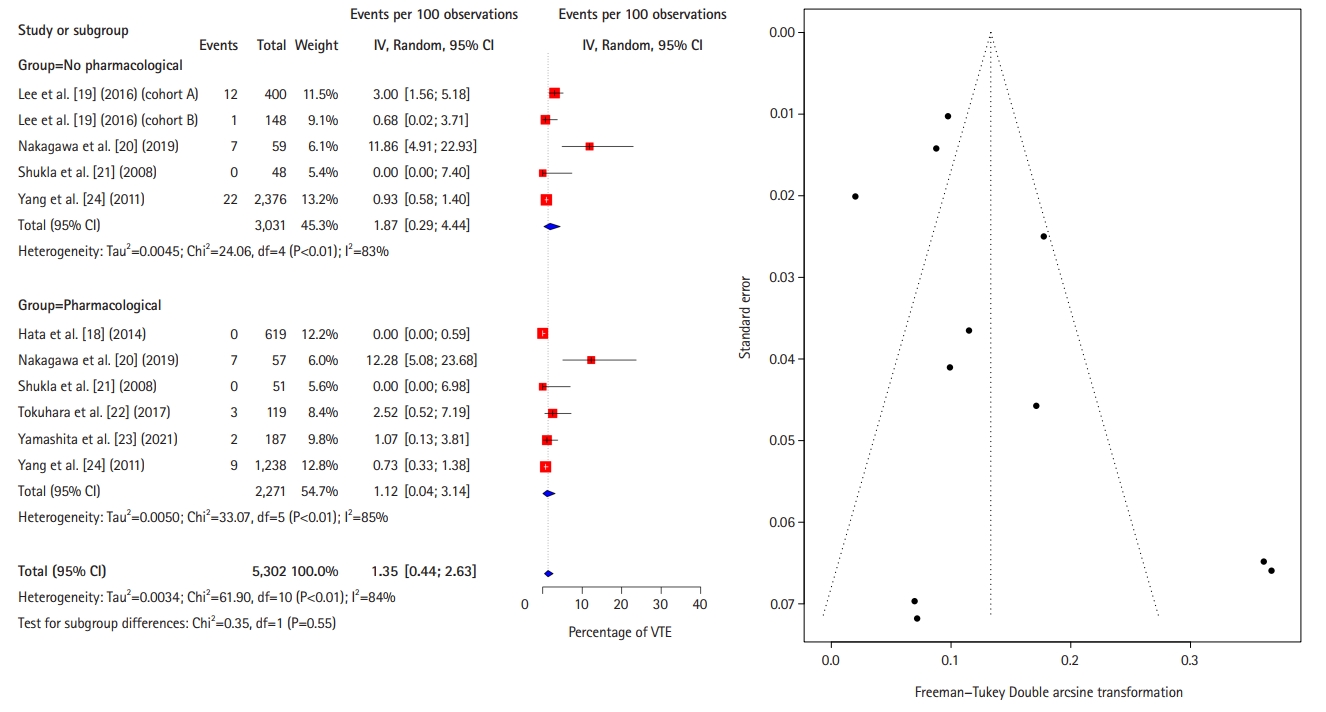
Fig. 3.
Forest plot comparing the incidence of symptomatic venous thromboembolism (VTE) between pharmacological thromboprophylaxis and no pharmacological thromboprophylaxis. IV, interval variable; Random, random-effects model; CI, confidence interval.

Fig. 4.
Forest plot comparing the incidence of proximal deep venous thrombosis between pharmacological thromboprophylaxis and no pharmacological thromboprophylaxis. IV, interval variable; Random, random-effects model; CI, confidence interval; VTE, venous thromboembolism.
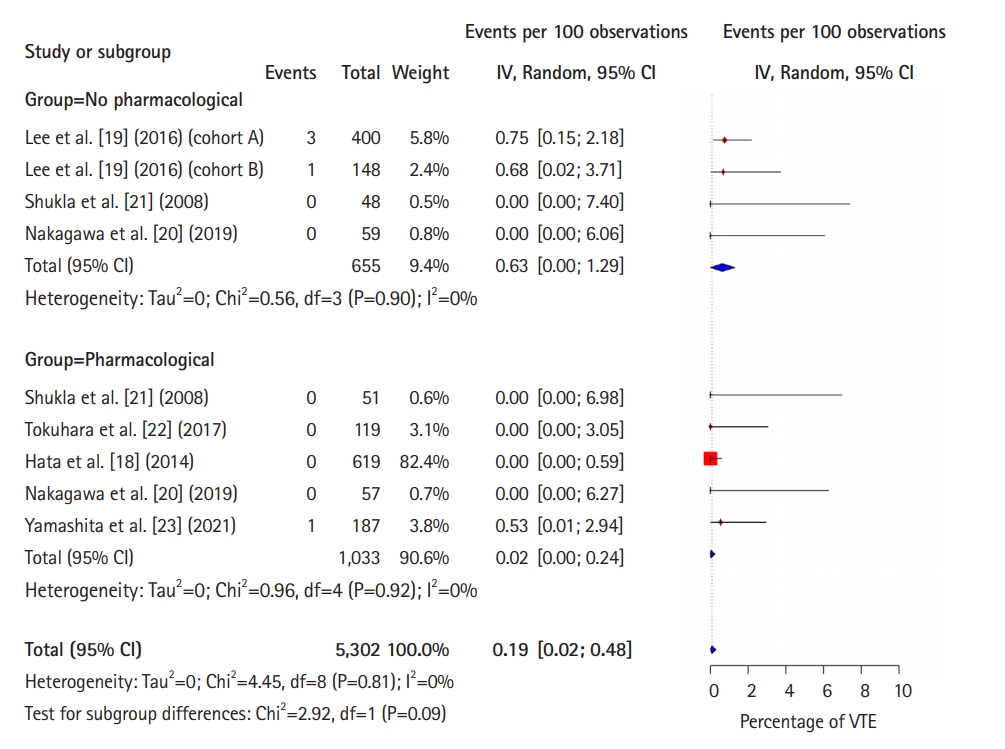
Fig. 5.
Forest plot comparing the incidence of pulmonary embolism between pharmacological thromboprophylaxis and no pharmacological thromboprophylaxis. IV, interval variable; Random, random-effects model; CI, confidence interval; VTE, venous thromboembolism.
Table 1.
Overview of the included studies
| Study | Year | Country | Study type | Quality score | No. of patients |
Sex |
BMI (kg/m2) | Laparoscopic (%) | PTP | Study design | Observational period | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | |||||||||||
| Lee et al. [19] (cohort A) | 2016 | Korea | Cohort | 22 | 400 | 248 | 152 | 23±3 | 69.8 | Not applicable | All patients had routine duplex US | POD 28 |
| Lee et al. [19] (cohort B) | 2016 | Korea | Cohort | 22 | 148 | 79 | 69 | 23±3 | 80.4 | Not applicable | Only symptomatic patients had venous US | POD 28 |
| Hata et al. [18] | 2014 | Japan | Cohort | 23 | 619 | 371 | 248 | 22±3 | 67.7 | Fondaparinux 2.5 or 1.5 mg | Only symptomatic patients had duplex US, MDCT, or ascending phlebography for DVT; MDCT, pulmonary scintigraphy, or pulmonary arteriography for PE | 1 Day after use of fondaparinux (4–8 day) |
| Nakagawa et al. [20] | 2019 | Japan | RCT | 27 | 116 | 55 | 61 | 22 (17–36)a | 100 | Enoxaparin 20 mg | All patients had routine duplex US | POD 28 |
| 23 (16–33)a | ||||||||||||
| Shukla et al. [21] | 2008 | India | RCT | 16 | 99 | 65 | 34 | 19b | 90.9 | Dalteparin sodium 2,500 IU | All patients had routine duplex US | POD 6/7 |
| Tokuhara et al. [22] | 2017 | Japan | Cohort | 20 | 119 | 78 | 50c | 22 (16–30)c | 100 | Fondaparinux 2.5 or 1.5 mg | Patients with a D-dimer score greater than 1 μg/mL on day 2 and day 7 postoperative underwent duplex US | POD 10 |
| Yang et al. [24] | 2011 | Korea | Cohort | 19 | 3,645 | 2,294 | 1,351 | NRd | NR | Enoxaparin 20 mg subcutaneous only to high-risk patients | Only symptomatic patients had duplex US or contrast venography for DVT; CT scan, ventilation-perfusion scan for PE | NR |
| Yamashita et al. [23] | 2021 | Japan | Cohort | 21 | 187 | 123 | 93c | NRc,e | 87.2 | Intravenous heparin 10,000 IU and enoxaparin 2,000 U subcutaneous | Only symptomatic patients had duplex US | POD 29 |
Values are presented as number only, mean±standard deviation, or median (interquartile range).
BMI, body mass index; PTP, pharmacological thromboprophylaxis; POD, postoperative day; US, ultrasonography; MDCT, multidetector computed tomography; DVT, deep vein thrombosis; PE, pulmonary embolism; RCT, randomized controlled trial; NR, not recorded; CT, computed tomography.
REFERENCES
1. Seddighzadeh A, Shetty R, Goldhaber SZ. Venous thromboembolism in patients with active cancer. Thromb Haemost 2007;98:656–61.


2. Imamura H, Adachi T, Kitasato A, Tanaka T, Soyama A, Hidaka M, et al. Safety and efficacy of postoperative pharmacologic thromboprophylaxis with enoxaparin after pancreatic surgery. Surg Today 2017;47:994–1000.



3. Yanagita T, Kusanagi H. Safety and effectiveness of enoxaparin as venous thromboembolism prophylaxis after gastric cancer surgery in Japanese patients. Am Surg 2016;82:1232–7.



4. Anderson DR, Morgano GP, Bennett C, Dentali F, Francis CW, Garcia DA, et al. American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv 2019;3:3898–944.




5. Fleming F, Gaertner W, Ternent CA, Finlayson E, Herzig D, Paquette IM, et al. The American Society of Colon and Rectal Surgeons clinical practice guideline for the prevention of venous thromboembolic disease in colorectal surgery. Dis Colon Rectum 2018;61:14–20.


6. Law Y, Chan YC, Cheng SW. Epidemiological updates of venous thromboembolism in a Chinese population. Asian J Surg 2018;41:176–82.


7. Nakamura M. Japanese guidelines for prevention of venous thromboembolism. J Jpn Soc Clin Anesth 2004;24:480–7.

8. Tran HN, Klatsky AL. Lower risk of venous thromboembolism in multiple Asian ethnic groups. Prev Med Rep 2019;13:268–9.



9. Jang MJ, Bang SM, Oh D. Incidence of venous thromboembolism in Korea: from the Health Insurance Review and Assessment Service database. J Thromb Haemost 2011;9:85–91.


10. Sakuma M, Nakamura M, Yamada N, Ota S, Shirato K, Nakano T, et al. Venous thromboembolism: deep vein thrombosis with pulmonary embolism, deep vein thrombosis alone, and pulmonary embolism alone. Circ J 2009;73:305–9.


11. Lee CH, Lin LJ, Cheng CL, Kao Yang YH, Chen JY, Tsai LM. Incidence and cumulative recurrence rates of venous thromboembolism in the Taiwanese population. J Thromb Haemost 2010;8:1515–23.


12. Liu HS, Kho BC, Chan JC, Cheung FM, Lau KY, Choi FP, et al. Venous thromboembolism in the Chinese population: experience in a regional hospital in Hong Kong. Hong Kong Med J 2002;8:400–5.

13. Molina JA, Jiang ZG, Heng BH, Ong BK. Venous thromboembolism at the National Healthcare Group, Singapore. Ann Acad Med Singap 2009;38:470–8.


14. Huerta C, Johansson S, Wallander MA, García Rodríguez LA. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med 2007;167:935–43.


15. Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrøm J. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost 2007;5:692–9.


16. Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med 1998;158:585–93.


17. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–84.



18. Hata T, Yasui M, Murata K, Okuyama M, Ohue M, Ikeda M, et al. Safety of fondaparinux to prevent venous thromboembolism in Japanese patients undergoing colorectal cancer surgery: a multicenter study. Surg Today 2014;44:2116–23.




19. Lee E, Kang SB, Choi SI, Chun EJ, Kim MJ, Kim DW, et al. Prospective study on the incidence of postoperative venous thromboembolism in Korean patients with colorectal cancer. Cancer Res Treat 2016;48:978–89.




20. Nakagawa K, Watanabe J, Ota M, Suwa Y, Suzuki S, Suwa H, et al. Efficacy and safety of enoxaparin for preventing venous thromboembolic events after laparoscopic colorectal cancer surgery: a randomized-controlled trial (YCOG 1404). Surg Today 2020;50:68–75.



21. Shukla PJ, Siddachari R, Ahire S, Arya S, Ramani S, Barreto SG, et al. Postoperative deep vein thrombosis in patients with colorectal cancer. Indian J Gastroenterol 2008;27:71–3.

22. Tokuhara K, Matsushima H, Ueyama Y, Nakatani K, Yoshioka K, Kon M. Efficacy and safety of thromboembolism prophylaxis with fondaparinux in Japanese colorectal cancer patients undergoing laparoscopic surgery: a phase II study. Int J Surg 2017;42:203–8.


23. Yamashita S, Nishi M, Ikemoto T, Yoshikawa K, Higashijima J, Tokunaga T, et al. Clinical analysis of postoperative venous thromboembolism in Japanese patients after colorectal cancer surgery. Surg Today 2021;51:1022–7.



24. Yang SS, Yu CS, Yoon YS, Yoon SN, Lim SB, Kim JC. Symptomatic venous thromboembolism in Asian colorectal cancer surgery patients. World J Surg 2011;35:881–7.



25. Liew NC, Alemany GV, Angchaisuksiri P, Bang SM, Choi G, De Silva DA, et al. Asian venous thromboembolism guidelines: updated recommendations for the prevention of venous thromboembolism. Int Angiol 2017;36:1–20.


26. Becattini C, Rondelli F, Vedovati MC, Camporese G, Giustozzi M, Boncompagni M, et al. Incidence and risk factors for venous thromboembolism after laparoscopic surgery for colorectal cancer. Haematologica 2015;100:e35–8.



27. Holwell A, McKenzie JL, Holmes M, Woods R, Nandurkar H, Tam CS, et al. Venous thromboembolism prevention in patients undergoing colorectal surgery for cancer. ANZ J Surg 2014;84:284–8.


28. Sanderson B, Hitos K, Fletcher JP. Venous thromboembolism following colorectal surgery for suspected or confirmed malignancy. Thrombosis 2011;2011:828030.




29. Kanchanabat B, Stapanavatr W, Meknavin S, Soorapanth C, Sumanasrethakul C, Kanchanasuttirak P. Systematic review and meta-analysis on the rate of postoperative venous thromboembolism in orthopaedic surgery in Asian patients without thromboprophylaxis. Br J Surg 2011;98:1356–64.



30. Mismetti P, Laporte S, Darmon JY, Buchmüller A, Decousus H. Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg 2001;88:913–30.



31. Turpie AG, Bauer KA, Caprini JA, Comp PC, Gent M, Muntz JE, et al. Fondaparinux combined with intermittent pneumatic compression vs. intermittent pneumatic compression alone for prevention of venous thromboembolism after abdominal surgery: a randomized, double-blind comparison. J Thromb Haemost 2007;5:1854–61.


32. Caprini JA, Arcelus JI, Laubach M, Size G, Hoffman KN, Coats RW 2nd, et al. Postoperative hypercoagulability and deep-vein thrombosis after laparoscopic cholecystectomy. Surg Endosc 1995;9:304–9.



33. Iwade M, Iwade K, Nomura M, Ozaki M. Differences in perioperative coagulation between Japanese and other ethnic groups undergoing laparoscopic cholecystectomy. Surg Endosc 2003;17:2012–5.



34. Gregg JP, Yamane AJ, Grody WW. Prevalence of the factor VLeiden mutation in four distinct American ethnic populations. Am J Med Genet 1997;73:334–6.


35. Ridker PM, Miletich JP, Hennekens CH, Buring JE. Ethnic distribution of factor V Leiden in 4047 men and women: implications for venous thromboembolism screening. JAMA 1997;277:1305–7.


36. Moubayed SP, Eskander A, Mourad MW, Most SP. Systematic review and meta-analysis of venous thromboembolism in otolaryngology-head and neck surgery. Head Neck 2017;39:1249–58.



37. Bene NC, Minasian RA, Khan SI, Desjardins HE, Guo L. Ethnic disparities in thrombotic and bleeding diatheses revisited: a systematic review of microsurgical breast reconstruction across the East and West. J Reconstr Microsurg 2022;38:84–8.


38. Ramanan B, Gupta PK, Sundaram A, Lynch TG, MacTaggart JN, Baxter BT, et al. In-hospital and postdischarge venous thromboembolism after vascular surgery. J Vasc Surg 2013;57:1589–96.


39. Population Divison. World population prospects 2019. United Nations; 2019 [cited 2021 Feb 7]. Available from: https://www.un.org/development/desa/pd/news/world-population-prospects-2019-0
- TOOLS








