Exfoliate cancer cell analysis in rectal cancer surgery: comparison of laparoscopic and transanal total mesorectal excision, a pilot study
Article information
Abstract
Purpose
Minimally invasive surgery (MIS) is currently the standard treatment for rectal cancer. However, its limitations include complications and incomplete total mesorectal excision (TME) due to anatomical features and technical difficulties. Transanal TME (TaTME) has been practiced since 2010 to improve this, but there is a risk of local recurrence and intra-abdominal contamination. We aimed to analyze samples obtained through lavage to compare laparoscopic TME (LapTME) and TaTME.
Methods
From June 2020 to January 2021, 20 patients with rectal cancer undergoing MIS were consecutively and prospectively recruited. Samples were collected at the start of surgery, immediately after TME, and after irrigation. The samples were analyzed for carcinoembryonic antigen (CEA) and cytokeratin 20 (CK20) through a quantitative real-time polymerase chain reaction. The primary outcome was to compare the detected amounts of CEA and CK20 immediately after TME between the surgical methods.
Results
Among the 20 patients, 13 underwent LapTME and 7 underwent TaTME. Tumor location was lower in TaTME (7.3 cm vs. 4.6 cm, P=0.012), and negative mesorectal fascia (MRF) was more in LapTME (76.9% vs. 28.6%, P=0.044). CEA and CK20 levels were high in 3 patients (42.9%) only in TaTME. There was 1 case of T4 with incomplete purse-string suture and 1 case of positive MRF with dissection failure. All patients were followed up for an average of 32.5 months without local recurrence.
Conclusion
CEA and CK20 levels were high only in TaTME and were related to tumor factors or intraoperative events. However, whether the detection amount is clinically related to local recurrence remains unclear.
INTRODUCTION
Like many surgical fields, laparoscopic or robotic minimally invasive surgery (MIS) is now accepted as the standard method for rectal cancer treatment as per large-scale randomized clinical trials (RCTs) [1]. However, since surgery for mid to low rectal cancer proceeds to a narrow and deep pelvis, the surgical field gradually moves away from the operator over time. It is often difficult to access a straight camera and surgical instruments through laparoscopy. The transanal total mesorectal excision (TaTME), a bottom-up approach, has been used for over 10 years to overcome this limitation [2]. It is performed with better margin and specimen quality than the laparoscopic approach, attracting the attention of many colorectal surgeons [3].
However, TaTME is not as widespread as conventional laparoscopic surgery for 2 main reasons. First, we are unfamiliar with the anatomical surgical field approached from the bottom. Second, there is concern regarding the leakage of tumor cells that may exist in the intestinal tract due to the approach from the anus. Especially in a national TaTME registry study conducted in Norway, an unusual pattern of early local recurrence was reported in many patients, and the study was suspended [4, 5].
TaTME is an inside to outside approach of the intestinal tract; thus, it may lead to the risk of leakage of tumor cells or contamination of the abdominal cavity by intestinal bacteria. To verify this hypothesis, objective and scientific verification of leakage through prospective sample collection may be required, but no research has been reported regarding this. To verify the risk of tumor cell leakage and bacterial contamination in the TaTME method, we conducted a preliminary study by collecting lavage fluid before and after TME in TaTME and laparoscopic TME.
METHODS
Ethics statement
This study was approved by the Institutional Review Board of the National Cancer Center of Korea (No. NCC2020-0143). Informed consent was obtained from all patients before surgery, and clinical information, surgical records, and pathological records were collected.
Patients
We consecutively recruited patients of all ages planning elective surgery with MIS from a single institution (National Cancer Center, Goyang, Korea) with biopsy-proven rectal adenocarcinoma from June 2020 to January 2021. Clinical staging was confirmed by performing chest computed tomography (CT), abdominopelvic CT, and rectal magnetic resonance imaging (MRI), with the disease located <15cm from the anal verge (AV) from colonoscopy. Patients with synchronous colon cancer or other cancers, metastatic colorectal cancer, emergency operation, perforation, obstruction, open surgery or open conversion, and local excision were excluded. In addition, for locally advanced rectal cancer with cT3–4N0-2, a multidisciplinary approach was used to determine the need for neoadjuvant chemoradiotherapy. The basic regimen included oral capecitabine and long course radiotherapy, followed by surgery 6 to 8 weeks after treatment.
Surgical procedure
All surgeries were performed as MIS, and some patients were randomly assigned because they were enrolled in the RCT comparing laparoscopic TME (LapTME) and TaTME [6]. For other patients, surgeons autonomously decided the surgical method based on the condition of the tumor. The surgery was performed by 2 senior surgeons with over 100 TaTME experiences.
Generally, the ligation level of the inferior mesenteric artery and vein was determined by the operator's judgment through laparoscopy, and splenic flexure mobilization was determined by considering the tension of the anastomosis. Upon TME and distal resection completion, proximal resection was performed using an extracorporeal method, followed by colorectal or coloanal anastomosis. Colorectal anastomosis was performed using a circular stapler (25–29 mm; CDH, Ethicon). A single pathologist evaluated the resection margin and TME quality of surgical specimens [7].
LapTME employed a laparoscopic instrument throughout the entire procedure. After mesorectal dissection to the distal end of the tumor, distal resection was performed using a linear stapler (Signia Stapling System or Endo GIA Roticulator, Covidien). If the tumor was close to the anus, only distal resection was performed through a transanal approach.
TaTME was defined as a case where over 50% of TME was performed with a transanal approach. The tumor is visually checked at the start, and a purse-string suture is performed at approximately 1 cm distal from the lesion. If the distal tumor margin was located approximately 3 cm from the anorectal junction, a purse-string suture was directly performed openly, the GelPOINT Path TAMIS Platform (Applied Medical) was mounted, and mesorectal dissection was started. When the lesion was located more than 3 cm from the anorectal junction, the GelPOINT Path was installed first, and a purse-string suture was performed with a single port followed by dissection.
Sample collection
Lavage samples were collected in 3 steps. At the start of the operation, after checking the inside of the abdominal cavity through a laparoscopy, 100 mL of normal saline was used toward the pelvis for peritoneal washing, and it was withdrawn with 2 syringes of 50 mL in the Douglas cavity (collect 1: before TME, 100 mL). Moreover, immediately after completion of TME, 200 mL of normal saline was sprayed at the dissection area using the instrument shown in Fig. 1, and lavage fluid was withdrawn using 3 syringes of 50 mL. One syringe was sent to a microbiological laboratory for bacterial culture, and 2 syringes were used for analysis (collect 2: after TME, 100 mL). After that, the dissection area was repeatedly and sufficiently washed with 500 mL or more of normal saline at the operator's discretion, and the last 50 mL were withdrawn (collect 3: after irrigation, 50 mL). After sample collection, proximal bowel resection was performed, or surgery was continued for patients requiring lateral lymph node dissection.
RNA extraction analysis
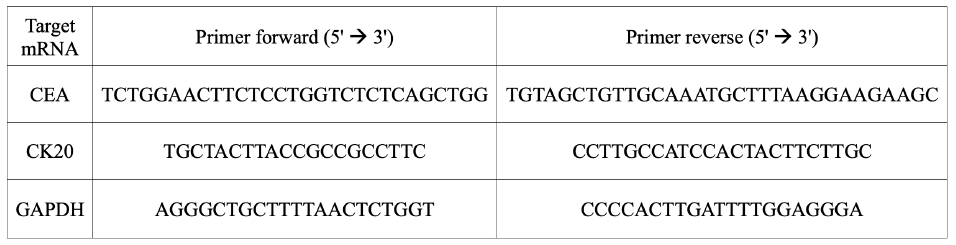
Each sample was stored at 4 °C immediately upon acquisition and sent to the laboratory after surgery completion. All samples were centrifuged at 1,200 rpm for 10 minutes at room temperature, the supernatant was removed, and the remaining collected pellet was prepared. To analyze expression levels of carcinoembryonic antigen (CEA) and cytokeratin 20 (CK20), total RNA was purified from the collected pellet using TRIzol Reagent (Thermo Fisher Scientific). After separating the aqueous phase, purification was performed using the RNeasy Mini Kit (Qiagen). Total RNA (100 ng) was reverse transcribed using RNA to cDNA EcoDry Premix–Oligo dT (Clontech Laboratories Inc) according to the manufacturer's instructions. Finally, 5 ng of complementary DNA was analyzed for expression analysis. CEA and CK20 messenger RNA (mRNA) expression levels were analyzed using the relative quantitative real-time polymerase chain reaction (qRT-PCR) method, and gene expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (primer sequences in Fig. 2). The qRT-PCR was performed on an LC480II RT-PCR using SYBR Green MasterMix (Roche Applied Science) in triplicate, and the PCR was performed as follows; 5 minutes at 95 °C for initial denaturation, followed by 45 cycles at 95 °C for 10 seconds, at 60 °C for 10 seconds, and at 72 °C for 10 seconds; finally, melting curve analysis was performed at 95 °C for 5 seconds, at 65 °C for 1 minute followed by cooling at 40 °C for 30 seconds.
Statistical analysis
The primary outcome of this study was to compare the detection amount of CEA and CK20 according to the 2 surgical methods and to determine the risk factors associated with exfoliating cancer cells during TME. Furthermore, as secondary outcomes, the detection amount before and after TME was compared, the effect of sufficient irrigation was checked, and bacterial contamination during TME and short-term clinical outcomes were evaluated.
The Mann-Whitney U-test and chi-square test (or Fisher exact test) were used to compare continuous and categorical variables between the 2 groups. A P-value of <0.05 was considered significant. All statistical analyses were performed using R ver. 4.2.0 (R Foundation for Statistical Computing).
RESULTS
Study population and comparison to the surgical method
From June 2020 to January 2021, 20 consecutive patients were diagnosed with adenocarcinoma of rectal cancer. Among the 20 patients, 13 patients underwent LapTME and 7 patients underwent TaTME. Table 1 shows the comparison between the 2 groups. There was no statistical difference between the 2 groups regarding sex, age, body mass index, and preoperative CEA levels. The median tumor height was lower in the TaTME group (7.3 cm vs. 4.6 cm from AV, P=0.012). There was no difference in extramural vascular invasion (EMVI) evaluated by preoperative MRI between the 2 groups. However, the mesorectal fascia (MRF) status was more negative in the LapTME group (76.9% vs. 28.6%, P=0.044). The number of patients who received neoadjuvant chemoradiotherapy (nCRT) before surgery was 100% in the TaTME group compared to 5 patients (38.5%) in the LapTME group. The overall operative time was longer in the TaTME group (median, 283 minutes vs. 366 minutes; P=0.047). In the anastomosis method, the ratio of coloanal type was high in the TaTME group, but there was no statistically significant difference (P=0.104). In the pathological examination results, tumor size, T category, lymph node status, TME completeness, margin status, and known risk factors were not significantly different between the 2 groups. Moreover, bacterial cultures showed negative results in all patients immediately after TME.
Relative detection CEA and CK20 levels in 3 steps
Relative quantification was performed by comparing the detection amount with GAPDH, a housekeeping gene, for CEA and CK20 in samples collected throughout 3 timepoints: before TME (collect 1), after TME (collect 2), and after irrigation (collect 3). The results are presented in Table 2. There was no detectable amount in collect 1 obtained by peritoneal washing before TME. In collect 2, which was obtained by washing immediately after TME, CEA and CK20 levels were high in 3 patients only in the TaTME group. Compared to other patients, the CEA:GADPH ratio and CK20:GADPH ratio were relatively higher in 3 patients: 1.39 and 1.11, respectively, for patient 1; 2.93 and 0.27, respectively, for patient 2; and 1.01 and 1.00, respectively, for patient 14 (Fig. 3A, B). Finally, collect 3, obtained after sufficient irrigation, showed a low detection amount of ≤0.1 in all patients (Fig. 3C, D).

Relative detection amount of carcinoembryonic antigen (CEA) and cytokeratin 20 (CK20) by surgical method in collect 2 (after total mesorectal excision [TME]) and collect 3 (after irrigation). (A) Collect 2 CEA. (B) Collect 2 CK20. (C) Collect 3 CEA. (D) Collect 3 CK20. The numbers indicate the patient number. LapTME, laparoscopic TME; TaTME, transanal TME; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Individual patient
Supplementary Table 1 presents specific information on the 20 patients who participated in this study. Eight out of 20 patients simultaneously participated in different RCTs and were randomly assigned a surgical method. Five patients underwent lateral lymph node dissection (LLND) after TME, and this procedure did not affect sample collection. Patients 1, 2, and 14 showed high CEA or CK20 levels and commonly underwent TaTME after nCRT (Table 3).
Patient 1, a 53-year-old male, had a 1 cm tumor located at AV 5.7 cm; the patient was positive for EMVI and threatened MRF on preoperative MRI. The time duration of the operation was 344 minutes, wherein the transanal approach lasted for 120 minutes, and the estimated blood loss (EBL) was 50 mL. An intraoperative event occurred during TaTME with small amounts of intestinal discharge due to purse-string failure (Fig. 4A). Postoperative pathology was the only confirmed T4; there was no lymph node metastasis, the distal resection margin (DRM) was 38 mm, and the circumferential resection margin (CRM) was 5 mm. The TME quality evaluated by the pathologist was nearly complete.

Intraoperative event. (A) Purse-string failure observed in patient 1. Discharge was observed from the center of the suture during dissection. (B) Dissection failure observed in patient 2. Mucin spillage occurred during mesorectal dissection.
Patient 2, a 63-year-old male with a 5 cm tumor located at the AV 2 cm, was negative EMVI and positive MRF on preoperative MRI. The operation lasted 468 minutes, including the time for LLND; the transanal time was 250 minutes, and there was 100 mL of EBL. During TaTME, there was a mucin spillage event at the 10 o'clock position due to dissection failure (Fig. 4B). The pathology results revealed T3N1, complete TME quality, DRM of 5 mm, and CRM of 1.2 mm.
Patient 14, a 46-year-old female, had a 1.5 cm tumor located at AV 6 cm; the patient was negative EMVI and negative MRF on preoperative MRI. The operation lasted 422 minutes, including the LLND time duration; the transanal time was 90 minutes, and there was 50 mL of EBL. However, there was no notable event that occurred during the transanal approach. The pathology results revealed T2N0, complete TME quality, DRM of 13 mm, and CRM of 9 mm.
Clinical outcomes
The median postoperative hospitalization period was 8 days (range, 5–20 days), and 1 patient (patient 5) showed Clavien-Dindo grade III or higher complications within 30 days. The patient underwent LapTME, and an anastomotic leak occurred on the 6th day after surgery; thus, a colostomy was performed, and stoma closure was done after adjuvant treatment. According to the stage and patient's condition, 14 patients (70%) received adjuvant treatment. Patients with an ileostomy underwent stoma repair between 2 to 7 months after surgery, except for 1 patient with a presacral abscess.
Planned follow-up was conducted at 1, 2, 3, and 6 months after surgery and thereafter at 6-month intervals. All patients were followed up for an average of 32.5 months (range, 24.8–37.5 months) without local recurrence and death. During this period, 2 laparoscopically operated patients had distant metastasis to the lung (patient 15, 30 months; patient 19, 25 months), and the complications were incisional hernia (patient 8) and presacral abscess due to delayed anastomotic leakage (patient 10) in the LapTME group.
DISCUSSION
Surgery for mid to low rectal cancer using a conventional laparoscopic approach is difficult due to anatomical features and the limitations of straight surgical instruments. The risk of complications is particularly high in known risk factors such as male sex, obesity, narrow pelvis, and large tumors [8]. Moreover, according to a meta-analysis [9], the risk of incomplete mesorectal excision is higher in laparoscopic surgery compared to open surgery. To overcome this problem, TaTME was started in 2010. Many colorectal surgeons around the world try this method with interest.
Although positive long-term results have been reported [10], there are concerns regarding serious problems such as early local recurrence and intra-abdominal bacterial contamination [4, 5, 11, 12]. Therefore, in this study, 20 patients were recruited to determine the difference between LapTME and TaTME by quantitatively and indirectly measuring the presence of cancer cells that shed in the dissection field during TME through peritoneal lavage. Consequently, it was confirmed that 3 out of 7 patients in the TaTME group had high values of CEA or CK20.
The most important feature of TaTME, which is differentiated from conventional LapTME, is to directly check the tumor through the anus, secure the distal margin, perform a purse-string suture, and start the operation from the inside to the outside of the rectum. The important pathologic findings included low positive DRM and CRM, and good or comparable quality of TME [10, 13–16]. Regarding the oncological outcome, local recurrence was reported in approximately 10% of TME alone and approximately 5% of TME following nCRT [17]. However, during a follow-up period of 19.5 months in Norway, 7.9% of patients with local recurrences and multifocal patterns were reported in 67%. Moreover, in the Netherlands, among the 10% of patients with local recurrences, also 67% had a multifocal pattern during the follow-up period of 21.9 months [11]. The recurrence of an unusual pattern in a short period raises a safety issue.
Considering intra-abdominal bacterial contamination, it is reported that 40% of 23 patients were culture positive, and half of them developed presacral abscess [12]. This could be attributed to the nature of initiating TME with an intraluminal approach. Exfoliated cancer cells or fecal leakage may occur due to incomplete purse-string suture or incorrectly entering the dissection plane, which may spread to the surrounding area due to pneumoperitoneum during laparoscopic surgery, air insufflation through the anus, and manipulation during surgery, but scientific and objective verification has not yet been made.
Therefore, we planned to analyze the samples collected through peritoneal lavage. However, there is no consensus on collecting and analyzing colorectal cancer, unlike gastric and ovarian cancer, where cytology is included in staging. Considering the findings of the previous studies, the lavage, which is the sample collection step, differs in the fluid type, amount, temperature, collection location, and collection timing [18]. In our study, samples were collected in 3 steps using warm saline. At the beginning of the operation, peritoneal washing was performed in the Douglas cavity using 100 mL of water (collect 1). Furthermore, immediately after TME, the most important sample, i.e., 200 mL of water, was sprayed on the dissection field, 100 mL were collected for analysis (collect 2), and another 50 mL were used for bacterial culture. Finally, sufficient irrigation was performed to collect 50 mL of clean-looking fluid (collect 3).
Regarding the analysis method, there have been many previous studies using cytology; recently, studies on the clinical significance of colorectal cancer through immunofluorescence or qRT-PCR are being conducted [18, 19]. The detection rate through conventional cytology for peritoneal lavage is about 13% (range, 2%–52%) [20], and immunofluorescence using various monoclonal antibodies reported a higher detection rate than that of cytology [21, 22]. In the qRT-PCR method, several studies reported clinical significance through quantitative analysis using CEA and CK20 as target mRNAs. A 28% to 42% positive rate was reported depending on the cutoff value [22–26]. After each analysis method's strengths and limitations were considered, several factors such as surgical method, tumor factors, and intraoperative events were expected to affect the results; thus, we planned the analysis using a quantitative method.
A relatively high value was shown in 3 out of 7 patients in the TaTME group but none in the LapTME group. Of these, 1 patient was identified as T4 in the pathological result, and there was a purse-string failure event during the operation; the other patient was MRF-positive on the preoperative MRI and had an intraoperative dissection failure event. The last patient had no identifiable risk factors or intraoperative events. In the ongoing COLOR (Colorectal Cancer Laparoscopic or Open Resection) III RCT [6], T4 and MRF ≤1 mm are the exclusion criteria. These tumor factors can affect the study outcomes related to surgical methods; thus, they are usually included in the exclusion criteria. However, as a preliminary study on the analysis method, the exclusion criteria were minimized, and 2 patients had tumor factors and intraoperative events simultaneously, which should be considered in interpreting the results.
In terms of introducing new technologies, TaTME has reported several learning curve studies. According to the results of 1,594 patients in the international registry reported by Penna et al. [27], approximately 30% of patients had intraoperative adverse events; a conversion rate of 5.6% and anastomotic failure of 15.7% were reported. Moreover, in the initial 720 cases examined by the same team [14], 0.6% of purse-string failures and 7.8% of the incorrect plane were reported. These events can potentially affect postoperative complications or long-term results such as local recurrence. Therefore, a considerable level of surgical expertise is needed in a very narrow surgical field. Accordingly, various institutions report the learning curve results concerning the operation time, intraoperative event, specimen quality, and complications [28–30]. To overcome this learning curve, an anatomical approach, technical analysis [31], and a systematic educational curriculum are presented and applied to show that TaTME can be safely performed in terms of intraoperative events and pathological outcomes [13, 32].
Bacterial culture was performed at the same time points as in the collect 2 step, but there was no significant finding. Although presacral abscess was not observed in pelvis CT performed within 1 to 4 weeks after surgery, anastomotic leakage was observed in 1 patient in the LapTME group. However, the relationship with intra-abdominal contamination is unknown. Meanwhile, patient 1 showed a small amount of watery stool leakage due to purse-string failure, but the reported culture result was negative. Therefore, this method is likely to have lower sensitivity than the study that employed the swab method [12].
After TME, sufficient irrigation was performed, and the amounts of CEA and CK20 detected in collect 3 were significantly reduced. Irrigation was performed using a larger amount of fluid than usual, and its effect could be confirmed with objective values. Thus, it is expected to prevent some adverse results caused by exfoliating cancer cells and bacterial contamination during surgery.
This study had some limitations. First, it was conducted as a preliminary study, the number of patients participating was small, and only a descriptive analysis of events was possible. Although there was one patient without any specified risk factor (patient 14), the reason for the elevated value could not be found. Second, as mentioned above, the tumor factor was not excluded during patient recruitment. Consequently, risk factors could not be distinguished because intraoperative events were also present in patients with significant detection values. Third, CEA and CK20 levels may be elevated by other causes [33]; thus, there is insufficient evidence to correlate increased levels of CEA and CK20 in our study subjects with oncological outcomes. However, considering local recurrence, this is a valuable pilot study in terms of the research method that compared the 2 surgical methods and quantitatively evaluated them.
Furthermore, there was no local recurrence during the mean follow-up period of 32.5 months. Although TaTME has a beneficial aspect as an approach for tumors close to the anus, it should be used cautiously for expanding indications because safety verification is still lacking. It is expected that the results of the ongoing RCT (COLOR III trial) [6] will provide evidence in the future.
In conclusion, high levels of CEA and CK20 were detected in lavage fluids in more than 40% of the TaTME patients immediately after TME and were related to tumor factors or intraoperative events. It is unclear whether the detection amount is clinically related to local recurrence, and follow-up studies are needed.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study was supported by the National Cancer Center Career Development Awards 2020 and a grant from the National Cancer Center of Korea (No. NCC-2110201). This work was also supported by the Genomics Core Facility in the National Cancer Center of Korea.
Author contributions
Conceptualization: KY, DKS; Formal analysis: KY, JAH; Funding acquisition: KY, DKS; Investigation: KSH, CWH, BK, BCK; Project administration: SCP; Resources: DWL, SSP, SCP, JHO; Supervision: JHO; Writing–original draft: KY, JAH; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Additional information
The abstract was presented at the International Colorectal Research Summit (ICRS) 2021, hosted by The Korean Society of Coloproctology, in Seoul, Korea, and as a poster presentation at the 17th Scientific and Annual Conference of the European Society of Coloproctology (ESCP) held in September 2022, in Dublin, Ireland.
SUPPLEMENTARY MATERIALS
Supplementary Table 1.
Individual patient information
Supplementary materials are available from https://doi.org/10.3393/ac.2023.00479.0068.