Distribution and Impact of the Visceral Fat Area in Patients With Colorectal Cancer
Article information
Abstract
Purpose
The purposes of this study were to investigate the distribution of the visceral fat area (VFA) and general obesity and to compare visceral and general obesity as predictors of surgical outcomes of a colorectal cancer resection.
Methods
The prospectively collected data of 102 patients with preoperatively-diagnosed sigmoid colon or rectal cancer who had undergone a curative resection at Pusan National University Yangsan Hospital between April 2011 and September 2012 were reviewed retrospectively. Men with a VFA of >130 cm2 and women with a VFA of >90 cm2 were classified as obese (VFA-O, n = 22), and the remaining patients were classified as nonobese (VFA-NO, n = 80).
Results
No differences in morbidity, mortality, postoperative bowel recovery, and readmission rate after surgery were observed between the 2 groups. However, a significantly higher number of harvested lymph nodes was observed in the VFA-NO group compared with the VFA-O group (19.0 ± 1.0 vs. 13.5 ± 1.2, respectively, P = 0.001).
Conclusion
Visceral obesity has no influence on intraoperative difficulties, postoperative complications, and postoperative recovery in patients with sigmoid colon or rectal cancer.
INTRODUCTION
In the United States of America, the prevalence of obesity is 35.5% among adult men and 35.8% among adult women, and there are approximately 1 billion overweight people worldwide [1]. According to a recent report, obesity and visceral obesity are closely related to the development of colorectal cancer, as well as other metabolic complications [2]. In addition, a previous article showed that obese patients had unfavorable surgical outcomes, including longer operative time, increased postoperative complication rate, increased conversion rate, and prolonged hospital stay [3]. However, body mass index (BMI) has been widely used to express the degree of obesity, although it does not always adequately reflect the degree of visceral fat [4]. Thus, we used the visceral fat area (VFA) to reflect the abdominal obesity directly and wanted to compare our result with the distribution of the population-based fat area. The purpose of this study was to investigate the distribution of VFA and general obesity and to compare visceral and general obesity as predictors of surgical outcomes of a colorectal cancer resection.
METHODS
Patients
The prospectively collected data on patients with preoperatively-diagnosed sigmoid colon or rectal cancer who had undergone a curative resection at our hospital between April 2011 and September 2012 were reviewed retrospectively; 197 patients with unchecked metabolic profile or waist circumference were excluded, and 102 patients were enrolled in this study. The clinical parameters analyzed included the patients' characteristics, perioperative outcomes (operative times, estimated blood loss, numbers of lymph nodes harvested, conversion rate, and intraoperative complications), and postoperative outcomes (days to flatus, stool, and food [liquid diet] intake, postoperative length of hospital stay, postoperative complications, readmission, and reoperation). Patients were staged using the TNM 7th edition. Operative time was calculated as the time between the first incision and wound closure. Before surgery, all patients underwent a standard bowel preparation 24 hours before the surgery and received oral antibiotics consisting of metronidazole. Prophylactic intravenous antibiotics were administered at the time of anesthetic induction and discontinued within 24 hours. Water could be taken orally on the day of surgery. Feeding began if the patient did not have an ileus and started with a liquid diet, progressing to a soft diet on the following day.
Surgical procedure
Surgery was performed by a surgical team consisting of 2 colorectal surgeons. Pneumoperitoneum was maintained at 10–12 mmHg. Dissection was performed using a medial-to-lateral approach, and the mesenteric artery was divided at the level of the root of the inferior mesenteric artery or distal to the origin of the left colic artery, according to the extent of disease. The splenic flexure was taken down if necessary in order to achieve an adequate resection margin and to perform a tension-free anastomosis. An end-to-end anastomosis was performed using a double-stapling technique with a circular stapler; however, the transanal hand-sewn technique was used during a laparoscopic transabdominal transanal proctosigmoidectomy. The integrity of the anastomosis was verified by using an air-leak test, followed by placement of a pelvic drain in the vicinity of the anastomosis through the left lateral port site. All patients were scheduled for follow-up at seven days after discharge.
Quantification of the area of visceral fat
In the current study, all patients underwent an abdominal computed tomography (CT) scan for preoperative assessment of the extent of the disease. The CT scanner is linked to a networked medical imaging system through which images are transferred electronically to a centralized data system and then retrieved at a workstation (Rapidia 2.8 software, INFINITT Co., Seoul, Korea). Software enables rendering of multiple images and geometric measurements of a specific region with a specified CT number (in Hounsfield units) (Fig. 1). A single cross-sectional scan at the level of the umbilicus was selected for quantification [45]. Adipose tissue was determined by setting the attenuation level within the range of –190 to –30 Hounsfield units, and the acquired image corresponded to the total fat region [67]. Finally, the VFA was calculated by using the software. All quantifying procedures described above were performed by a single examiner, who was blinded to the surgical outcome at the time of quantification.

Quantification of the visceral fat area on the computed tomography (CT) scan. (A) Original CT scan image at the level of the umbilicus. (B) The visceral fat region is determined by outlining the intra-abdominal component and the subcutaneous fat region. (C) Alteration of the window width for detection of adipose tissue.
Definition of obesity
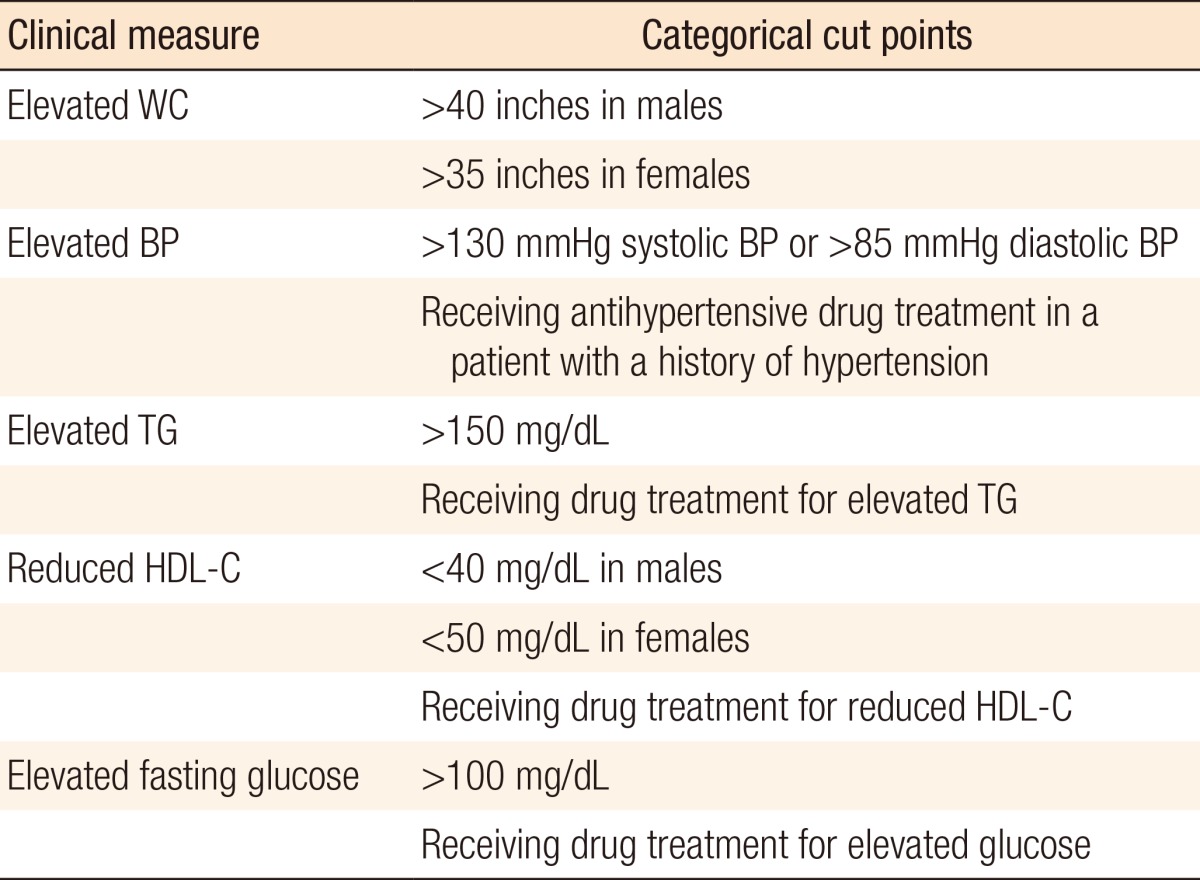
Several methods have been proposed for estimating the visceral obesity [489]. In this study, we divided patients into 2 groups according to the cutoff levels of VFA recommended by Oka et al. [10]. These levels take into account both sex-related differences and the quantity of visceral fat in Japanese. Men with a VFA ≥130 cm2 and women with a VFA ≥90 cm2 were classified as obese (VFA-O), and the remaining patients were classified as nonobese (VFA-NO). BMI was calculated, and obese patients were defined as those with BMI ≥25 kg/m2 (BMI-O), and the remaining patients as nonobese (BMI-NO), in accordance with the Asia-Pacific Perspective: Redefining Obesity and its treatment proposed area-specific cut points [8]. Each patient in this study underwent preoperative screening for metabolic syndrome (MS). Diagnosis of MS was based on the 2005 American Heart Association and the National Heart, Lung, and Blood Institute criteria, which included the presence of three or more of the following parameters: waist circumference, blood pressure, triglyceride level, high-density lipoprotein cholesterol level, and blood glucose level (Table 1) [11]. As there are no standard values for the definition of normal VFA/SFA ratio, we divided patients according to the percentiles of their VFA/SFA ratio [12].
Statistical analysis
Statistical analysis was performed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). Numeral variables are expressed as mean ± standard deviations or mean (range) and were compared using the Mann-Whitney U-test or the Student t-test. Categorical data were tabulated as percentages and compared using the Pearson chi-square test or Fisher exact probability test. Correlations between VFA and other obesity indices (BMI, MS, WC, and VFA/SFA ratio) were examined using a one-way analysis of variance, and Pearson product-moment coefficient (r) was used to assess the correlations between obesity indices. Finally, the VFA and other obesity indices were examined for association with intraoperative and postoperative complications, reoperation, and readmission by using a multivariate model of logistic regression. A P-value of less than 0.05 was considered statistically significant.
RESULTS
Characteristics of patients relative to visceral obesity
This study included 102 patients with sigmoid colon or rectal cancer with an average age of 62 years (range, 32–85 years), of whom 65% were male. There were 22 VFA-O patients (21.6%) and 80 VFA-NO patients (78.4%). Fifty-four patients (18.1%, 54 of 299) had MS. The demographic and the clinical characteristics of the 102 patients are shown in Table 2. Between the 2 groups, VFA-NO and VFA-O, no significant differences in age, American Society of Anesthesiologists score, preoperative carcinoembryonic antigen levels, or operative procedure were noted. VFA-O patients were more likely to be female than VFA-NO patients were. The pathologic data on the patients with or without visceral fat obesity are shown in Table 2. A specific difference in the numbers of retrieved nodes between the VFA-NO and VFA-O groups (19 ± 1.0 vs. 13.5 ± 1.2, P = 0.001) was noted.
Distribution of visceral obesity and the relationship between the area of visceral fat and other obesity indices
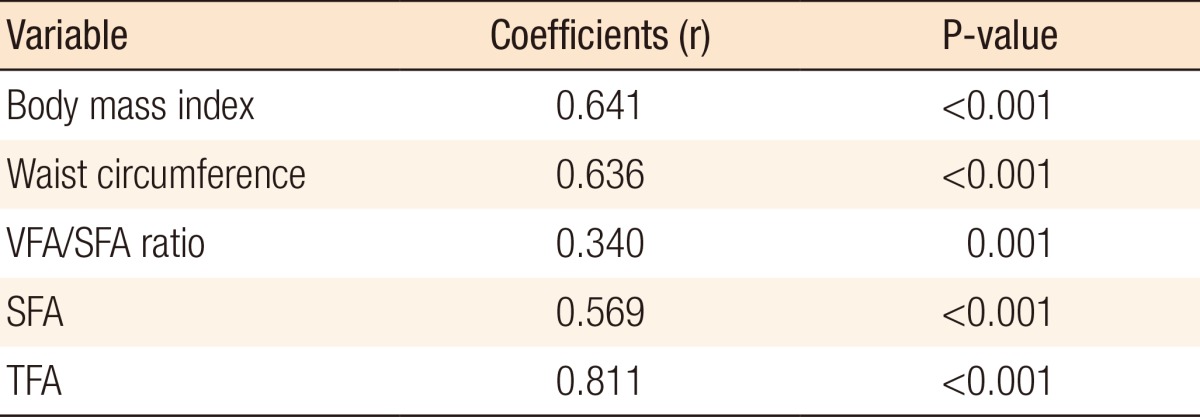
In order to examine the relationship between general obesity and the VFA in our sample, we tested the correlations between the VFA and different obesity indices (Table 3). We found a high correlation of the VFA with BMI (r = 0.614, P < 0.001) (Fig. 2), WC (r = 0.636, P < 0.001), VFA/SFA ratio (r = 0.340, P = 0.001), SFA (r = 0.569, P < 0.001), and TFA (r = 0.811, P < 0.001). To examine the distribution of visceral obesity, we tested the comparison of obesity indices according to age. As people grow older, the VFA increases; however, the SFA decreases. The VFA/SFA ratio showed a statistically significant difference with age (Table 4).
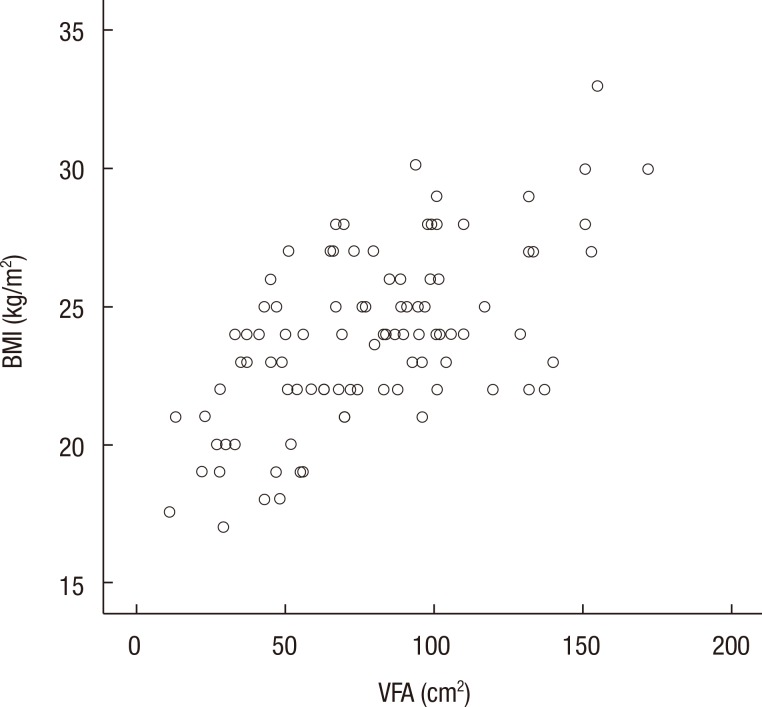
Scatter plots showing the correlation between the visceral fat area (VFA) and the body mass index (BMI).
Surgical outcomes of patients relative to visceral obesity
Intraoperative variables used for assessing the technical difficulty are shown in Table 5. The intraoperative morbidity was 5.0% in the VFA-NO group and 4.5% in the VFA-O group. The difference did not reach statistical significance. The conversion rate was 2.5% in the VFA-NO group, and the reasons were intractable adhesions in the context of obesity and a bulky tumor.
The postoperative outcomes used for assessing the short-term outcomes are shown in Table 5. The postoperative morbidity was 42.5% in the VFA-NO group and 31.8% in the VFA-O group, including drain infection in 1 patient, dermatitis in 2, chylous ascites in 3, delirium in 1, pancreatitis in 2, leg numbness due to epidural block in 1, and dizziness in 1. There was no mortality in this study. No significant difference in time to recovery of postoperative gastrointestinal function was observed between the VFA-NO and the VFA-O groups. There were 5 readmissions. In 2 cases, voiding difficulty and dizziness were managed nonoperatively. One patient experienced gastritis and another patient had a wound infection. In 1 patient, anastomotic insufficiency was managed in the operating room by aseptic irrigation and drainage because he had undergone a diverting ileostomy. Two patients underwent a reoperation for anastomotic leakage on postoperative days 4 and 27, respectively. An anastomotic hemorrhage was resolved with surgical suture ligation in 1 patient.
Correlation of obesity with operative outcomes (Table 6)

Risk factors for intraoperative complication, postoperative complication, reoperation, and readmission based on the multivariate analysis
A multivariate analysis was performed in order to estimate the correlation of obesity with operative outcomes (intraoperative complication, postoperative complication, readmission, and reoperation). The risk for intraoperative complications was not significant; neither were the risks associated with BMI, WC, VFA, and VFA/SFA ratio. However, MS was on obvious risk for postoperative complications (P = 0.017; odds ratio, 2.987). In the multivariate analysis, the risks for readmission and reoperation did not reach statistical significance.
DISCUSSION
In this study, we investigated the surgical outcomes for viscerally obese patients with colorectal cancer. No significant differences in outcomes were observed between the obese and the nonobese patients. Obesity, a growing health problem worldwide, is usually defined using the BMI. In particular, increasing BMI has been associated with colorectal carcinogenesis [1314]. Besides general obesity, which is expressed by the BMI, the distribution of adipose tissue also seems to be important in the pathogenesis of colorectal cancer [15]. Increased visceral obesity, measured by using the VFA and the VFA/SFA ratio, has been examined as a predictor of colorectal surgery outcome [41216]. In those studies, visceral obesity was associated with prolonged operative time [16], increased postoperative complications [216], shorter disease-free survival [12], and longer hospital stay [2] after a resection for colorectal cancer.
Although the BMI has been considered one of the most reliable anthropometric indices of obesity, several techniques have recently been developed for assessing the VFA from a single CT scan obtained at the level of the umbilicus. The VFA has been shown to correlate closely with the total volume of visceral fat [617]. In addition, accumulating evidence has suggested a strong association between visceral obesity and MS, as discussed in recent reviews [1819]. Lohsiriwat et al. [2] reported a higher rate of in patients with MS than in patients without MS.
In our study, visceral obesity had no influence on intraoperative difficulties, postoperative complications, and postoperative recovery in patients with colorectal cancer, but did have an influence on the number of harvested lymph nodes. The number of harvested lymph nodes, with resection margin status, is a measure of the oncologic adequacy of the resection. In addition to a low average lymph node yield of 9.5, Ballian et al. [20] found an association of increasing visceral obesity with reduced retrieval of lymph nodes. Moon et al. [12] suggested a lack of association between the VFA/SFA ratio and the number of lymph nodes harvested in 161 patients undergoing a laparoscopic resection for colorectal cancer. However, in that study, only 59% of patients had rectal cancer, and the VFA/SFA ratio was measured at the level of the iliac crests and umbilicus. In their study of 141 proctectomy specimens, Gorog et al. [21] found that specimens from patients with BMI >25 kg/m2 and measuring <16 cm in length contained significantly fewer lymph nodes than those from patients with BMI <25 kg/m2. No significant differences in lymph node retrieval were observed in patients with specimens longer than 16 cm, nor did obese patients tend to have shorter proctectomy specimens. A significant difference in the number of harvested lymph nodes was observed between our two groups, the VFA-NO and the VFA-O groups. We speculate that these results were due to the variable cutoff value of the VFA; i.e., men with a VFA of ≥130 cm2 and women with a VFA of ≥90 cm2 were classified as obese. Although we divided patients into two groups according to the cutoff levels of the VFA recommended by Oka et al. [10], different criteria for different genders might induce differences in the surgical outcomes for patients with colorectal cancer. A second explanation for the similar operative outcomes is the observation that the rectal cancer tumors were larger in VFA-NO than in VFA-O, but the tumor locations were not statistically different.
As to the relationship between the VFA and other obesity indices, the current study showed high correlations of the VFA with the BMI, WC, and VFA/SFA ratio. However, if simplicity and opportunity costs are considered in the preoperative evaluation of patients' obesity, the method measuring the BMI and the WC might be a better way to assess obesity. On the distribution of visceral fat according to age, visceral obesity increased with age, but the SFA decreased. As a result, the VFA/SFA ratio increased until patients were in their 70s and decreased in patients aged 80 years and older. In addition, the VFA/SFA ratio showed statistical significance according to age. Furthermore, as described in other studies [2022], we may suppose that the BMI, the VFA/body surface area and the VFA/SFA ratio are more reliable as predictive factors of short-term surgical outcomes for patients with sigmoid colon or rectal cancer.
Ballian et al. [20] reported an association of visceral obesity with postoperative, oncologic, and survival outcomes of a TME for a rectal adenocarcinoma. There were no significant risks for intraoperative and postoperative complications, reoperation, or readmission, except for patient with MS (OR, 2.987; P = 0.017). In the current study, we postulated that visceral obesity would not affect the results of surgery because the mean BMI of our samples was 23.9 ± 0.33 kg/m2 and the number of morbidly obese patients was lower than that of Western people.
This study had several limitations. First, it was retrospective in design, although the data were collected prospectively. Second, our samples were small, and patients underwent various operative procedures, which increases the heterogeneity. Because the cutoff value of visceral obesity in women was lower, many more women were included in the VFA-O group.
In conclusion, we found a high correlation of VFA with waist circumference and the VFA/SFA ratio. As people grow older, the VFA increases, but the SFA decreases. In this study, we found that visceral obesity had no influence on intraoperative difficulties, postoperative complications, and postoperative recovery in patients with colorectal cancer.
Notes
The oral presentation at the Young Investigator Award of the Korean Surgical Society, Seoul, Korea, December 1, 2012.
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.