METHODS
The colonoscopy study group of the Korean Society of Coloproctology conducted a retrospective study of colorectal carcinoid tumors in collaboration with eight institutes from January 2006 to December 2009. We gathered data such as age, gender, site, size, invasion depth and the presence of metastasis through the collection and review of medical records.
Rectal carcinoids were defined as tumors located within 15 cm of the anal verge while tumors more than 15 cm above the anal verge were regarded as colonic carcinoids. The tumors were classified into three groups according to tumor size: 1) a tumor diameter less than 10 mm, 2) a 10-mm to 20-mm tumor diameter, and 3) a diameter larger than 20 mm. Tumor sizes were confirmed through pathology reports. In the case of tumor sizes not being clearly described in the pathology reports, the size was determined according to the colonoscopy reports. Treatment methods were classified as endoscopic resection, transanal resection and radical operation.
In cases treated by radical operation, the presence of lymph node metastasis was determined by using a pathology report while in cases treated by local resection, including colonoscopic removal, metastatic status were assessed by radiologic studies (computer tomography [CT] scan). Cases which did not undergo metastatic work ups were excluded from the analysis of lymph node metastasis. If there were synchronous carcinoid tumors, only the largest tumors were included in the analysis of lymph node metastasis.
The tumor disease stages were determined according to the AJCC Cancer Staging 7th edition, revised in 2010. Statistical analysis was done with the Fisher's exact test and a P-value less than 0.05 was considered to be statistically significant.
RESULTS
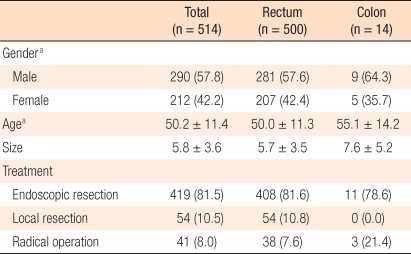
Five hundred two patients were treated for carcinoid tumors during the study period. In 12 patients, two carcinoid tumors were detected simultaneously (synchronous carcinoid tumor, 2.4%); thus, the total number of resected carcinoid tumors was 514. Fourteen carcinoid tumors were found in the colon, which accounts for 2.7%, and 500 carcinoid tumors (97.3%) were detected in the rectum. The ratio between males and females was 1.38:1, and the mean patient age was 50.2 years. The average tumor size was 5.8 mm, and a regional resection, including colonoscopic treatments, was performed in 473 cases (92%) (
Table 1).
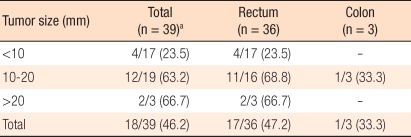
The result of pathological tests for 39 cases treated by radical surgery showed 18 cases of lymph node metastasis (46.2%). The relationship of lymph node metastasis to tumor size for tumors smaller than 10 mm was 23.5%, for tumors between 10 mm and 20 mm, it was 63.2%, and for tumors larger than 20 mm, it was was 66.7% (
Table 2).
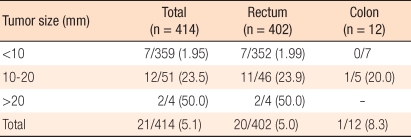
Metastasis was assessed through CT scans or pathological examinations in 414 cases. When cases showing metastasis findings on the test were considered as positive for lymph node metastasis, the aforementioned incidence according to tumor size for tumors smaller than 10 mm was 1.95% while for sizes between 10 mm and 20 mm, it was 23.5%, and for sizes larger than 20 mm, it was 50.0%. As tumor sizes increased, the metastasis incidence also increased (
Table 3), and the frequency of lymph node metastasis in cases with carcinoid tumors smaller than 10 mm was confirmed to be significantly lower than in other groups (P < 0.001). Regarding two cases with distant metastasis, one rectal case had metastasis to the liver, and the other colonic case involved peritoneal metastasis. The result of the TNM disease stage classification according to the AJCC is shown in
Table 4.
DISCUSSION
Generally, the incidence of carcinoid tumors of the colon has been reported to be very low and comprises 4-8% of carcinoid tumors in the digestive system [
1-
4]. Colonic carcinoid tumors are detected in the cecum primarily, and two-thirds of colonic carcinoid tumors have been reported to occur in the right side of the colon [
8]. More than half of carcinoid tumors of the colon are associated with lymph node metastasis or distant metastasis at the time of detection. Thus, prognosis is poor, with 5-year survival rates reported to be approximately 40-70% [
1,
9].
In our study, although it was limited to the colon and the rectum, the tumor incidence in the colon was 2.7%, which is lower than the generally accepted incidence rate. As well, the number of cases treated with radical surgery was only three. Among the three, one case had lymph node metastasis, and another case had peritoneal metastasis. For the remaining eleven cases, the tumors were resected locally through colonoscopy. Using these data, estimating the lymph node metastasis rate in terms of colonic carcinoid tumors is very difficult, making risk predictions infeasible.
The proportion of colonoscopic resections for colonic carcinoid tumors in our series was relatively high in relation to general treatment guidelines, which recommend radical surgery for all colonic carcinoids. A possible explanation for this is that contrary to the usual experience of finding colonic carcinoid tumors at advanced stages, many colonic tumors in our series may were found incidentally at smaller sizes during diagnosis, deeming colonoscopic resection appropriate.
Most carcinoid tumors we detected were in the rectum, correlating with known findings as to the organ in which carcinoid tumors are detected most frequently. In our study, cases involving rectal carcinoid tumors totaled 500, accounting for approximately 97.3%, with an average size of 5.7 mm. The frequency of lymph node metastasis for rectal carcinoid tumors was found to be 5.0%. Most rectal carcinoid tumors were treated by endoscopic or regional resection, such as transanal resection.
To evaluate the risk of metastasis, pathological examination of lymph nodes should be implemented. However, small rectal carcinoids are known to have little risk of metastasis, making local treatment desirable and reserving radical surgery for selected cases with risk factors associated with lymph node metastasis. Therefore, we substituted radiologic evaluation with CT scans for pathologic examination to confirm lymph node metastasis. Although CT scans do not reflect lymph node metastasis as accurately as a pathologic examination, if no sign of metastasis appears in the CT scan, we regard the case as negative for lymph node metastasis.
Four hundred four cases undergoing radical surgery and/or CT scan were included in the evaluation of metastasis. There was a direct correlation between tumor size and lymph node metastasis. The lymph node metastasis rate was approximately 2% if the tumor size was less than 10 mm and increased gradually up to 50% as the tumor size increased to larger than 20 mm. For tumor sizes between 10 mm and 20 mm, the risk of lymph node metastasis was as high as 24%, which was similar to the results reported by Soga et al. [
2]. This is thought to be a very important finding in deciding treatment modality. A consensus exists for which treatment method is appropriate for rectal carcinoid tumors with sizes between 10 mm and 20 mm.
Carcinoid tumors develop in the mucosal gland and gradually infiltrate to the lamina propria, submucosal layer and deeper layer of the bowel wall. As the disease stage advances, carcinoid tumors may be accompanied by lymph node or distant metastasis. These clinical behaviors are the characteristics of malignant neoplasms. Thus, it is reasonable to regard colorectal carcinoids as malignant tumors. In diverse reports, lymph node metastasis and distant metastasis were reported and are thought to be tumors with a malignant potential comparable to that of an adenocarcinoma [
2,
3]. In the recently revised AJCC cancer staging, carcinoid tumors are classified as a malignant disease. The stage classifications are based on the size of the tumor, the involved layer of the bowel wall and the presence or absence of lymph node or distant metastasis. According to this staging system, our series of cases are classified into 388 stage I cases, 4 stage IIA cases, no stage IIB or IIIA cases, 20 stage IIIB cases, and 2 stage IV cases. The staging results reflect that most cases were early lesions and were detected incidentally by screening colonoscopy.
In cases of rectal carcinoids, the risks of lymph node metastasis and distant metastasis vary depending on the tumor size, making tumor size the most important factor in deciding on a treatment method. The reported risk of lymph node metastasis for rectal carcinoids smaller than 10 mm is usually less than 3%; thus, regional treatment including colonoscopic resection is appropriate. However, radical surgery is recommended for tumors larger than 20 mm, where the risk of metastasis is known to be 60-80%. Treatment methods for tumors with sizes between 10 mm and 20 mm are controversial, and no established treatment guideline exists.
Known risk factors, other than tumor size, are tumor invasion beyond the proper muscle, the degree of cell differentiation and the mitotic rate. A population-based study in Japan reported 3.7% lymph node metastasis for rectal carcinoids with sizes of 5 mm and less and 10% for tumors with sizes less than 10 mm [
2]. These results complicate deciding on treatment methods even in small-sized rectal carcinoids. Therefore, if lymph node metastasis is to be predicted accurately, additional studies on risk factors are required, and treatment methods should be determined considering various risk factors [
9-
11].
The large number of cases involved in this study aided in revealing meaningful data in terms of the risk of lymph node metastasis, yet, some limitations should be mentioned. First, it was a retrospective cross-sectional study and collected data were restricted to findings when tumors were detected and treated. Second, there may have been selection bias. In some institutions, only cases involving radical surgery were included. These cases may have had risk factors associated with lymph node metastasis. Third, we could not estimate the overall incidence, and this study lacked long-term follow-up results in terms of recurrence. This may play a role in underestimating the risk of lymph node metastasis. Additional studies on recurrence and the rate of metastasis through long-term follow-up are required in the future.
In rectal carcinoid tumors, the size of the tumor is directly related to the frequency of lymph node metastasis. In carcinoid tumors with sizes smaller than 10 mm, the risk of lymph node metastasis was low; thus, it could be treated by regional treatments including colonoscopic resection. However, in carcinoid tumors with sizes larger than 10 mm, the risk of lymph node metastasis increased remarkably. Especially, rectal carcinoid tumors with sizes between 10 mm and 20 mm showed a lymph node metastasis rate of 23.5%, requiring treatment to be determined by considering various risk factors. Additional studies on the risk factors for lymph node metastasis are needed.