- Search
Abstract
Purpose
The aim of this study is to evaluate long-term survival and prognostic factors for radio-frequency ablation (RFA) in colorectal liver metastases.
Methods
We retrospectively reviewed 35 colorectal liver metastases patients who underwent RFA between 2004 and 2008. We analyzed survival after RFA and prognostic factors for survival.
Results
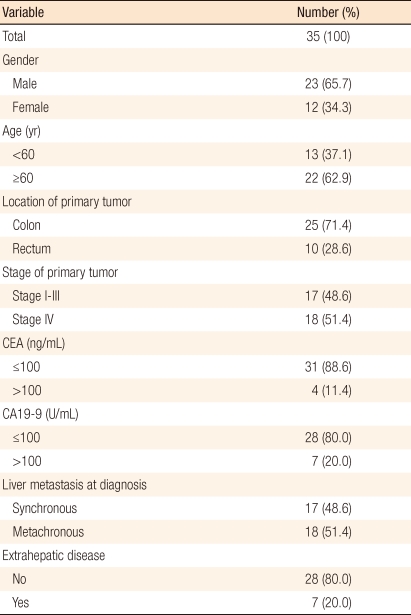
Of the 35 patients, 23 patients were male and 12 were female. Their mean age was 62.40 ± 12.52 years. Mean overall survival was 38.8 ± 4.6 months, and mean progression free survival was 19.9 ± 3.4 months. Three- and 5-year overall survival rates were 42.7 ± 0.1% and 26.0 ± 0.1%, respectively. Three- and 5-year progression-free survival rates were 19.6 ± 0.1% and 4.9 ± 0.04%, respectively. Overall survival and progression-free survival were significantly improved in male and in patients with carcinoembryonic antigen (CEA) ≤ 100 ng/mL, carbohydrate antigen (CA) 19-9 ≤ 100 ng/mL, absence of extrahepatic disease, and a unilobar hepatic lesion. In addition, progression-free survival was improved in patients with a solitary hepatic lesion. On the multivariate analysis, significant survival factors were the absence of extrahepatic disease and the presence of a unilobar hepatic lesion.
Colorectal cancer is one of the most common cancers worldwide [1] and is one of the main causes of death [2]. Metastasis occurs in about 50% of patients with colorectal cancers [3, 4], hepatic metastasis being the most common [5, 6]. Hepatic metastasis has been reported to be found in about 20-25% of patients with colorectal cancers at diagnosis. It occurs in about 20-30% of patients within the first 3 years of treatment for colorectal cancers [1]. The first line of treatment for hepatic metastases of colorectal cancers is surgical resection [1, 6-8], with a five-year survival rate of 31-58% [2]. However, the percentage of surgical resections for lesions in the liver at diagnosis is only 10-15%, and if a hepatic resection is not performed, the five-year survival rate is about 1-2% [7]. Therefore, chemotherapy, radio-frequency ablation, transarterial chemoembolization or percutaneous ethanol injection therapy has been attempted in patients on whom a hepatic resection cannot be performed [1, 2, 5].
Among the above treatments, radio-frequency ablation (RFA) is used for not only primary carcinomas but also metastatic carcinomas, and it is widely used for hepatic metastases of colorectal cancers in patients on whom a hepatic resection cannot be performed [7, 9]. RFA is an easily applied method because it is less invasive, and the risk of side effects or mortality is comparatively low compared to other treatment options, including surgery. Furthermore, RFA can be performed repeatedly in cases of local recurrence at the site after RFA or at a new metastatic hepatic carcinoma lesion. Even though RFA has often been performed thus far for primary or metastatic hepatic carcinomas in patients with fewer than 4 tumors, each being smaller than 4 cm, on whom a surgical resection could not be performed, the applicable targets of RFA have been broadened [7]. However, the results reported for these treatments are insufficient in spite of the expansion of applicable targets [2]. Thus, the purpose of this study is to investigate the factors that can affect the survival rate of patients with hepatic metastases of colorectal cancers after RFA treatment.
This study was retrospectively conducted on 35 patients with hepatic metastases of colorectal cancers. The patients were treated with RFA at Ewha Womans University Hospital from January 2004 to December 2008.
Inclusion criteria in our study were as follows: (1) complete resection of the hepatic lesion was impossible, (2) recovery was considered as difficult due to age, co-morbidities or poor health conditions existed even though a complete resection was possible, (3) a hepatic resection was not expected to lead to recovery of a patient with metastases of organs other than the liver, or (4) the patient refused surgery. However, patients were excluded from RFA (1) when a radical resection for the primary carcinoma was impossible, (2) when the lesion in the liver could not be detected through ultrasonography, (3) when a severe coagulation deficiency existed there were more than five metastatic lesions, or (4) when the largest lesion was bigger than 5 cm.
RFA was conducted through a percutaneous approach under local anesthesia, with some procedures being performed during surgery for colorectal cancer. The device used for treatment was a radio-frequency generator with a capacity of 200 watts (Valleylab, Boulder, CO, USA), and an electric current was emitted, targeting the tissue of the tumors, by using a unipolar radio-frequency electrode or expandable multi-electrode for 12-15 minutes. Response to treatment was evaluated using abdominal computed tomography one month after RFA. The results were considered as complete necrosis when contrast enhancement was not detected during the arterial phase or the portal phase at the treated lesion, and the tumor size was smaller. Clinical follow-up was performed every three months using computed tomography, and RFA was additionally conducted when a new tumor was found.
The effect of RFA on patients with hepatic matastases of colorectal cancers was evaluated by analyzing the overall survival and the progression-free survival rates. Clinical characteristics, including gender, age, and tumor marker and histopathological characteristics, such as location or clinical stage of the primary carcinoma and size and number of hepatic metastases, were investigated. The relationships between these characteristics and the survival rates were also analyzed.
The overall survival and the progression-free survival rates of the patients were analyzed using the Kaplan-Meier method, and the survival rates according to clinical or histological characteristics were compared using a univariate log-rank test and a multivariate Cox proportional hazards model. The statistical analysis was conducted using SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA) for Windows, and a P-value < 0.05 was considered as significant.
Out of the 35 patients, 23 were male and 12 were female, and their mean age was 62.40 ± 12.52 years (range, 35 to 81 years). Seventeen patients had synchronous hepatic metastases, 9 of whom were treated with RFA during surgery for the primary carcinoma and 7 with a percutaneous approach while hospitalized after surgery for the primary carcinoma; the remaining patient was treated with RFA after the size of the hepatic metastasis had been reduced through chemotherapy after surgery for the primary carcinoma due to the widespread hepatic metastases. Of these 17 patients, 3 had metastases of other organs, lung, ovary and peritoneum, respectively, besides the liver when diagnosed as having colorectal cancer. Eighteen patients had metachronous hepatic metastases, and the mean time to hepatic metastases was 16.27 ± 12.08 months (range, 2.03 to 50.4 months).
Before hepatic metastases had occurred, four patients had metastases of other organs, the lungs in two patients, the kidney in one patient, and the peritoneum in one patient. All 18 patients who had metachronous hepatic metastases were treated with RFA using the percutaneous approach a few days after the diagnosis of hepatic metastases (Table 1).
The primary locations of the carcinomas were the colon in 25 patients and the rectum in 10, and clinical stages were stage 1 in one patient, stage 2 in 4, stage 3 in 12, and stage 4 in 18 (Table 1). Out of these 18 patients at clinical stage 4, 16 had hepatic metastases, 1 had peritoneal metastasis, and 1 had concurrent hepatic and pulmonary metastases. The mean values of carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) before RFA were 47.19 ± 116.82 ng/mL (range, 1.3 to 561.1 ng/mL) and 1,021.63 ± 5,200.31 U/mL (range, 0.6 to 30381.4 U/mL), respectively.
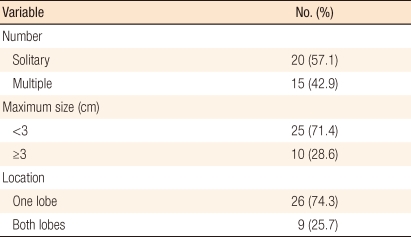
The mean number of hepatic metastases was 1.82 ± 1.20, and there were 20 patients with 1 hepatic metastasis, 8 patients with 2, 1 patient with 3, 5 patients with 4 and 1 patient with 5. The mean maximal diameter of the carcinomas was 2.42 ± 1.11 cm (range, 1 to 5 cm), and 26 patients had carcinomas with size < 3 cm and 9 with size ≥ 3 cm. Carcinomas were located at one lobe or both lobes of the liver in 26 and 9 patients, respectively (Table 2).
The mean number of RFAs conducted was 1.34 ± 0.64 (range, 1 to 3 cm). Twenty-six patients were treated with an ultrasonography-guided percutaneous approach, and 9 patients were treated during surgery for the primary carcinoma. There was no case that the hepatic resection performed prior to performing RFA. One patient presented with metastatic carcinomas at S4, 6, 7, and 8 and was treated at S4 while the hepatic resection was being performed on the right lobe.
Response to the treatment was evaluated using abdominal computed tomography after one month. Thirty-two patients showed complete necrosis of the carcinoma while 3 patients were treated with additional RFA due to incomplete necrosis. There were no significant side effects from RFA, and there were two cases of mild fever, one case each of hematoma and bleeding, and no cases of death related with RFA.
Clinical follow-up was performed using computed tomography every three months after treatment with RFA, and the mean follow-up period was 30.55 ± 21.56 months (range, 2.73 to 74.2 months). The mean progression-free survival duration was 19.9 ± 3.4 months (range, 2.2 to 74.2 months). The three-year and the five-year progression-free survival rates were 19.6 ± 0.1% and 4.9 ± 0.04%, respectively. In 32 patients (91.4%), recurrent hepatic carcinomas were detected during the clinical follow-up period, and a hepatic resection was performed on 2 of them; additional RFA was conducted on 7. Metastases occurred in other organs besides the liver in 18 patients (51.4%), with pulmonary and peritoneal metastases being the most common. The mean overall survival duration was 38.8 ± 4.6 months (range, 3.8 to 78.5 months), and the three-year and the five-year survival rates were 42.7 ± 0.1% and 26.0 ± 0.1%, respectively.
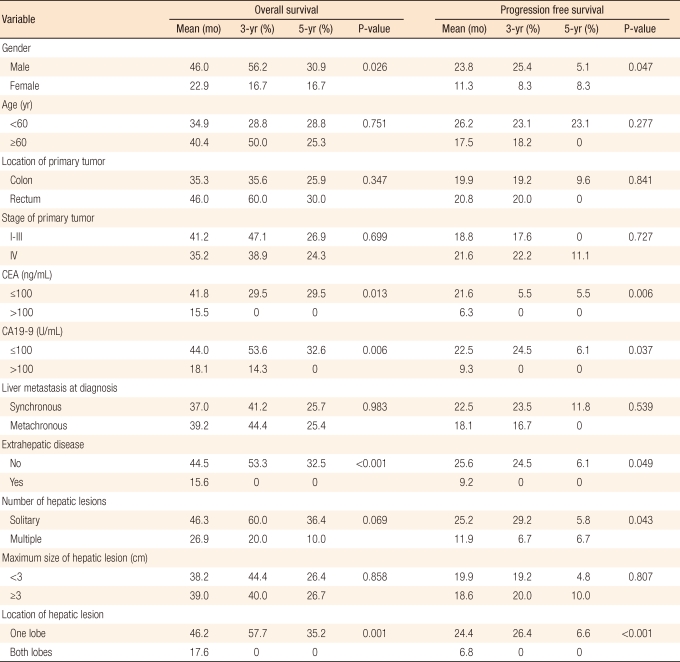
As a result of the univariate analysis of the dependence of the survival rates on the clinical and the histological characteristics of the patients, the progression-free survival rate was found to be significantly increased when the hepatic metastasis was a single carcinoma, and both the overall survival rates and the progression-free survival rates were found to be significantly increased when the patients were male, when CEA was ≤ 100 ng/mL or CA19-9 was ≤ 100 U/mL, when there were no metastases in organs other than the liver before RFA, and when the carcinoma was localized in only one lobe of the liver (Table 3, Figs. 1-6). In addition, based on a multivariate analysis of these factors and the survival rates, the overall survival rate was found to be significantly increased (P = 0.003) when there was no metastases in organs other than the liver before RFA, and the progression-free survival rate was found to be significantly increased when the carcinoma was localized in only one lobe of the liver (P = 0.045, P = 0.018) (Table 4). Other factors, such as age, location of the carcinoma, clinical stage of the carcinoma and time of hepatic metastases, did not significantly associated with the survival rates (Table 3).
RFA is one of the treatment modalities for localized hepatic cancers, and it necrotizes cancer cells due to increasing the temperature of tissues up to 70-100℃ with a radio-frequency current of 460-480 kHz for approximately 10 to 15 minutes after setting the electrodes at the target carcinoma [5, 10, 11]. RFA can be effectively used to treat primary and metastatic hepatic carcinomas because about 10% of their hepatic metastases are single carcinomas showing relatively slow progression [11]. Even though surgical resection is primarily considered in these cases, RFA is considered as a conservative method when surgery is not suitable due to age, systematically poor health, metastases of other organs besides the liver, or refusal of surgery by patients [11-13]. In addition, RFA is conducted when carcinomas are located at both lobes in the liver, when it is considered difficult to secure a negative resection margin, when carcinomas are located close to a large blood vessel, when the carcinoma is a recurrent metastatic carcinoma, or when the carcinoma is not resectable even after chemotherapy.
RFA is known to be a relatively safer treatment method than a hepatic resection or other localized treatment. In a study conducted by Rhim et al. on 1,139 patients, the mortality rate caused by RFA was reported as 0.09% and the side effect rate as 2.43% [14]. In another study by Livraghi et al. [15] on 2320 patients, the mortality rate was reported as 0.3%, the serious side effect rate as 2.2%, and the mild side effect rate as less than 5%. In other studies, mild side effects, such as pain, fever or hepatic abscess, were greater. Even though hepatic failure, tumor rupture, damage to blood vessels and the biliary tract, thrombosis, and propagation of tumor cells via electrodes occurred, their incidence rates were reported as low [11, 13] In this study, there were no serious side effects while there were two cases of mild fever (5.7%), one case each of hematoma and bleeding (2.8%) and no cases of death related to the treatment.
Studies to investigate the effects of RFA treatment have been constantly conducted since it was first initiated. However, investigations to study the effects of long-term use of RFA have been rare. Progression-free survival rates related to localized recurrence in the liver have been reported in only a few cases. In addition, studies of prognostic factors are insufficient. Thus, a purpose of this research was to evaluate over five years the effects of RFA by investigating the five-year overall survival rates and the progression-free survival rates and finding related prognostic factors in patients with hepatic metastases of colorectal cancers.
According to some studies on local recurrence after RFA, results vary from a low rate of recurrence at 3-4% [16, 17] to as high as 29-55% [18, 19]. The results reported in Korea showed relatively high rates at 48-53% [5, 6]. In this study, local recurrence was found in 32 patients (91.4%) out of 35 patients, and this high recurrence rate is considered to be affected mainly by selection bias of the target patients, such as size and number of tumors, degree of invasion in surrounding tissues, and the presence of micrometastases, by technical differences between the surgeons, and by the long duration of the follow-up.
Various results on survival rates after RFA of hepatic metastases of colorectal cancers have been reported, and the mean survival duration was 21 months. The two-year overall survival rate was 81.5% according to a report by Choi et al. [6] in Korea. The three-year and the five-year survival rates were 46-68% and 26-44%, respectively, in another study [19-21]. In a recent study conducted for 10 years, the mean survival duration was 24 months, the three-year overall survival rate was 20.2% and the five-year overall survival rate was 18.4% [22]. There was significant difference in this study compared to a previous study with the three-year overall survival rate at 42.7%, the five-year overall survival rate at 26.0%, and the three-year and the five-year progression-free survival rates at 19.6% and 4.9%, respectively.
Some results from studies on prognostic factors and survival rates for RFA have recently been reported. According to a study by Gillams et al. [2] on 309 patients with hepatic metastases of colorectal cancers, survival rates were significantly increased after RFA in cases where the number of tumors was ≤ 5, their maximal diameters were ≤ 5 cm, and there were no metastases besides the liver. Also, factors such as location and clinical stage of the primary carcinoma, regimen of chemotherapy and time to hepatic metastases did not show any significant relation to survival. Another study showed that the survival rate was clinically increased when the number of hepatic metastases was ≤ 3 and their maximal diameters were ≤ 3 cm each, or when CEA was ≤ 200 ng/mL [22]. As a result of a univariate analysis of prognostic factors related to survival rates in this study, the progression-free survival rate was significantly increased when the hepatic metastasis was a single lesion (P = 0.043). Both the overall survival rate and the progression-free survival rate were significantly increased when the patient was male (P = 0.026, P = 0.047), CEA was ≤ 100 ng/mL (P = 0.013, P = 0.006), and CA19-9 was ≤ 100U/mL (P = 0.006, P = 0.037). Also, when there were no metastases in organs besides the liver before RFA (P < 0.001, P = 0.049) or when the carcinoma was localized in only one lobe of the liver (P = 0.001, P < 0.001), the overall survival rate and the progression-free survival rate were significantly increased. Additionally in a multivariate analysis, patterns similar to those other studies were shown, where the overall survival rate was significantly increased when there were no metastases in organs besides the liver before RFA treatment (P = 0.003), and the overall survival rates and the progression-free survival rates were significantly increased when the carcinoma was located in only one lobe of the liver (P = 0.045, P = 0.018).
In our study, we suggests that RFA may be a safe, simple, and cost-effective treatment modality; the duration of hospital stay can be reduced even though cure cannot be expected in all patients with hepatic metastases of colorectal cancers. In addition, a great benefit of RFA is that it can be conducted repeatedly in cases of recurrence. This study showed no significant differences in overall survival rates and progression-free survival rates compared with other studies even though the recurrence rate was high, with results showing that gender, CEA, CA19-9, presence of other metastases besides liver, and number and location of tumors acted as prognostic factors. Even though the results of RFA were not compared with the results for hepatic resection on patients with hepatic metastases of colorectal cancers in this study, RFA isconsidered to be an option for treatment when the patient is male, CEA is ≤ 100 ng/mL, CA19-9 is ≤ 100 U/mL, no metastasis exists in organs other than the liver, the hepatic metastasis is a single lesion, and the carcinoma is localized in only one lobe of the liver. In conclusion, we suggest that RFA may be a good alternative for surgery with a possibly comparable survival rates for patients of old age and poor general condition, as well as for patients who refuse surgery.
References
1. Van Tilborg AA, Meijerink MR, Sietses C, Van Waesberghe JH, Mackintosh MO, Meijer S, et al. Long-term results of radiofrequency ablation for unresectable colorectal liver metastases: a potentially curative intervention. Br J Radiol 2011;84:556–565. PMID: 21159807.



2. Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol 2009;19:1206–1213. PMID: 19137310.


3. de Castro-Carpeño J, Belda-Iniesta C, Casado Sáenz E, Hernández Agudo E, Feliu Batlle J, González Barón M. EGFR and colon cancer: a clinical view. Clin Transl Oncol 2008;10:6–13. PMID: 18208787.


4. Yun SH. Molecular targeted therapy in colorectal cancer. J Korean Soc Coloproctol 2004;20:180–188.
5. Min BS, Lee KY, Park JK, Kim NK, Lee JT, Min JS. Radiofrequency ablation of hepatic metastasis from colorectal cancer; early experience. J Korean Surg Soc 2002;62:145–149.
6. Choi SI, Chang WY, Paik KY, Lee DS, Oh SH, Kim JH, et al. Short-term results of radiofrequency ablation for liver metastasis of colorectal cancer. J Korean Soc Coloproctol 2002;18:53–58.
7. Kang SJ, Park CM, Jeong KW, Park SB, Yun SH, Chang WY, et al. Clinical comparison of hepatic resection and radiofrequency ablation of hepatic metastases from colorectal cancer. J Korean Soc Coloproctol 2004;20:163–168.
8. Mulier S, Ruers T, Jamart J, Michel L, Marchal G, Ni Y. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? An update. Dig Surg 2008;25:445–460. PMID: 19212117.


9. Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004;239:818–825. PMID: 15166961.



10. Curley SA. Radiofrequency ablation of malignant liver tumors. Oncologist 2001;6:14–23. PMID: 11161225.


11. Hanna NN. Radiofrequency ablation of primary and metastatic hepatic malignancies. Clin Colorectal Cancer 2004;4:92–100. PMID: 15285816.


12. Liu LX, Jiang HC, Piao DX. Radiofrequence ablation of liver cancers. World J Gastroenterol 2002;8:393–399. PMID: 12046057.



13. Curley SA, Izzo F. Radiofrequency ablation of primary and metastatic hepatic malignancies. Int J Clin Oncol 2002;7:72–81. PMID: 12018113.


14. Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics 2003;23:123–134. PMID: 12533647.


15. Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radiofrequency ablation: complications encountered in a multicenter study. Radiology 2003;226:441–451. PMID: 12563138.


16. Pearson AS, Izzo F, Fleming RY, Ellis LM, Delrio P, Roh MS, et al. Intraoperative radiofrequency ablation or cryoablation for hepatic malignancies. Am J Surg 1999;178:592–599. PMID: 10670879.


17. Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg 2000;232:381–391. PMID: 10973388.



18. Bowles BJ, Machi J, Limm WM, Severino R, Oishi AJ, Furumoto NL, et al. Safety and efficacy of radiofrequency thermal ablation in advanced liver tumors. Arch Surg 2001;136:864–869. PMID: 11485520.


19. Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology 2001;221:159–166. PMID: 11568334.


20. Jakobs TF, Hoffmann RT, Trumm C, Reiser MF, Helmberger TK. Radiofrequency ablation of colorectal liver metastases: mid-term results in 68 patients. Anticancer Res 2006;26:671–680. PMID: 16739337.

21. Sorensen SM, Mortensen FV, Nielsen DT. Radiofrequency ablation of colorectal liver metastases: long-term survival. Acta Radiol 2007;48:253–258. PMID: 17453491.


22. Siperstein AE, Berber E, Ballem N, Parikh RT. Survival after radiofrequency ablation of colorectal liver metastases: 10-year experience. Ann Surg 2007;246:559–565. PMID: 17893492.


Fig. 1
Kaplan-Meier survival based on gender. (A) Males had improved overall survival compared with females (46.0 vs. 22.9 mo; P = 0.026). (B) Males had improved disease-free survival compared with females (23.8 vs. 11.3 mo; P = 0.047).
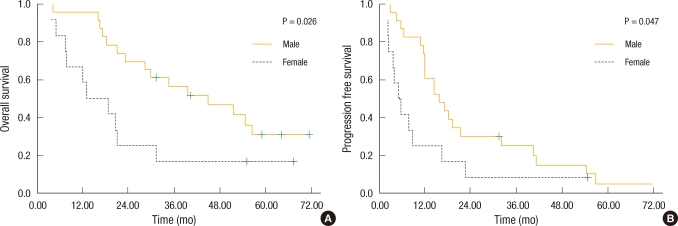
Fig. 2
Kaplan-Meier survival based on serum carcinoembryonic antigen (CEA) levels. (A) Patients with CEA ≤ 100 ng/mL had improved overall survival compared with those with CEA > 100 ng/mL (41.8 vs. 15.5 mo; P = 0.013). (B) Patients with CEA ≤ 100 ng/mL had improved progression-free survival compared with those with CEA > 100 ng/mL (21.6 vs. 6.3 mo; P = 0.006).
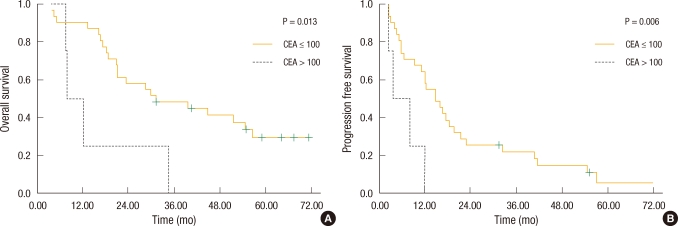
Fig. 3
Kaplan-Meier survival based on serum CA19-9 levels. (A) Patients with CA19-9 ≤ 100 U/mL had improved overall survival compared with those with CA19-9 > 100 U/mL (44.0 vs. 18.1 mo; P = 0.006). (B) Patients with CA19-9 ≤ 100 U/mL had improved progression-free survival compared with those with CA19-9 > 100 U/mL (22.5 vs. 9.3 mo; P = 0.037). CA, carcinoembryonic antigen; CEA, carcinoembryonic antigen.
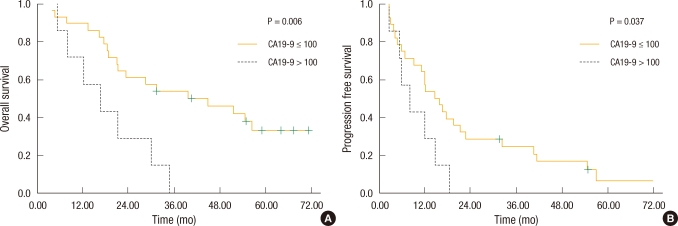
Fig. 4
Kaplan-Meier survival based on evidence of extrahepatic disease. (A) Patients without extrahepatic disease had improved overall survival compared with those with extrahepatic disease (44.5 vs. 15.6 mo; P < 0.001). (B) Patients without extrahepatic disease had improved progression-free survival compared with those with extrahepatic disease (25.6 vs. 9.2 mo; P = 0.049).

Fig. 5
Kaplan-Meier survival based on the number of hepatic lesions. (A) Patients with a solitary hepatic lesion had improved overall survival compared with those with multiple hepatic lesions (46.3 vs. 26.9 mo; P = 0.069). (B) Patients with a solitary hepatic lesion had improved disease-free survival compared with those with multiple hepatic lesions (25.2 vs. 11.9 mo; P = 0.043).
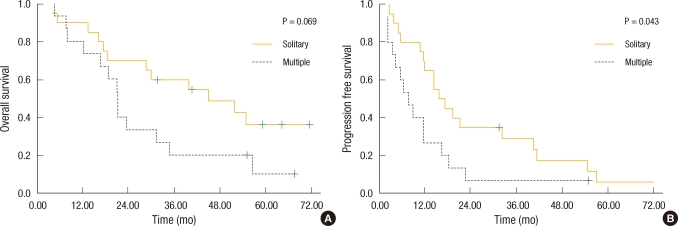
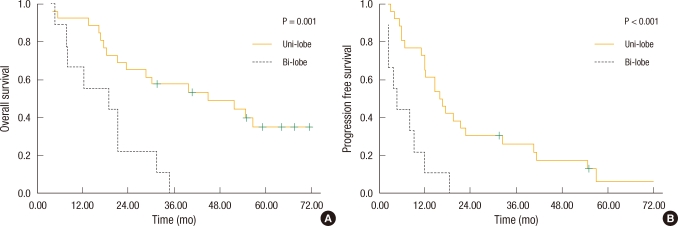
Fig. 6
Kaplan-Meier survival based on the location of the hepatic lesion. (A) Patients with a hepatic lesion in one lobe had improved overall survival compared with those with hepatic lesions in both lobes (46.2 vs. 17.6 mo; P = 0.001). (B) Patients with a hepatic lesion in one lobe had improved disease-free survival compared with those with hepatic lesions in both lobes (24.4 vs. 6.8 mo; P < 0.001).