Does the Different Locations of Colon Cancer Affect the Oncologic Outcome? A Propensity-Score Matched Analysis
Article information
Abstract
Purpose
We evaluate the prognostic value of primary tumor location for oncologic outcomes in patients with colon cancer (CC).
Methods
CC patients treated with curative surgery between 2009 and 2012 were classified into 2 groups: right-sided colon cancer (RCC) and left-sided colon cancer (LCC). Recurrence-free survival (RFS) and overall survival (OS) were examined based on tumor stage. Propensity scores were created using eight variables (age, sex, T stage, N stage, histologic grade, presence of lymphovascular invasion/perineural invasion, and microsatellite instability status).
Results
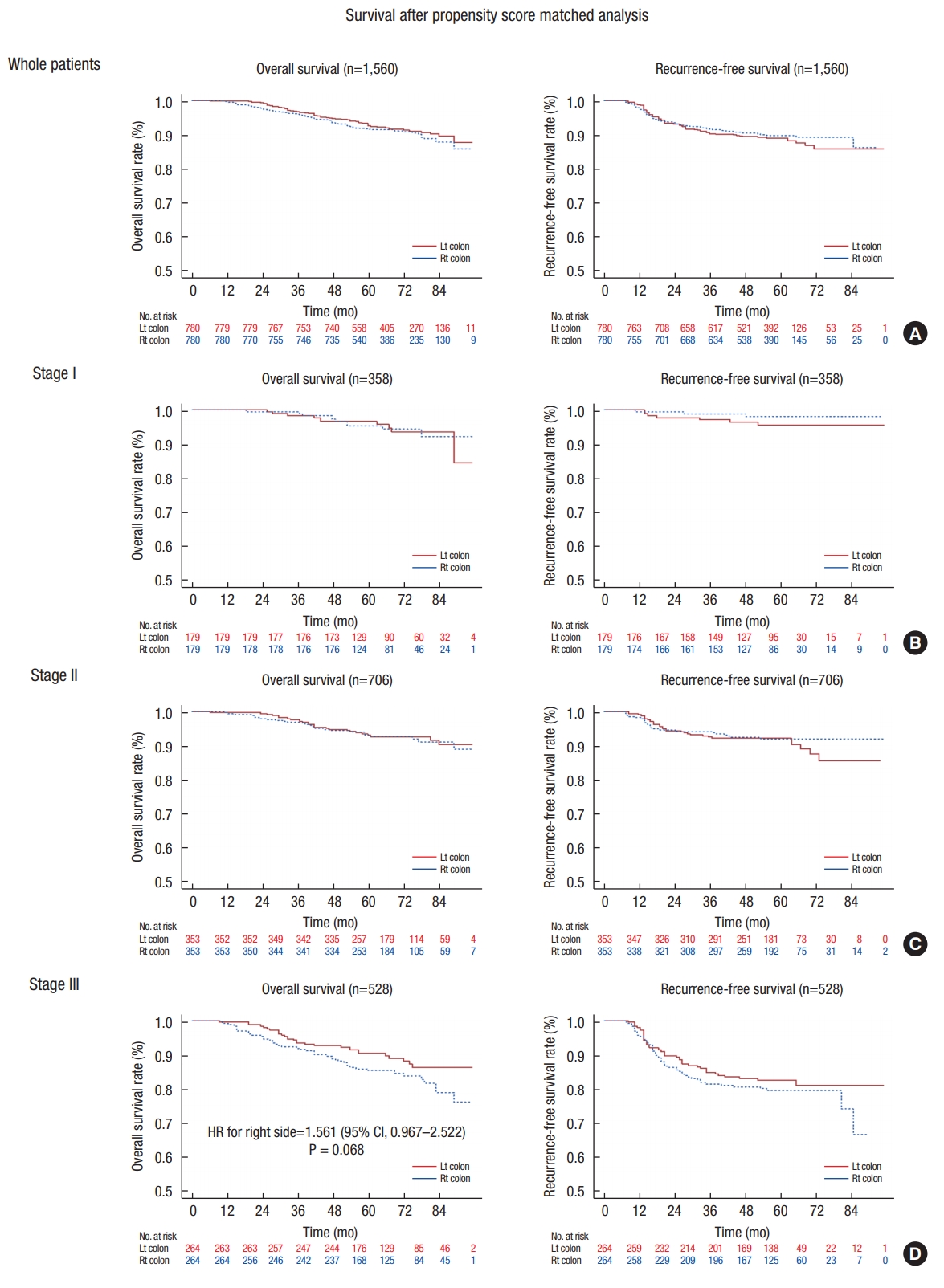
Overall, 2,329 patients were identified. The 5-year RFSs for RCC and LCC patients were 89.7% and 88.4% (P = 0.328), respectively, and their 5-year OSs were 90.9% and 93.4% (P = 0.062). Multivariate survival analyses were carried out by using the Cox regression proportional hazard model. In the unadjusted analysis, a marginal increase in overall mortality was seen in RCC patients (hazard ratio [HR], 1.297; 95% confidence interval [CI], 0.987–1.704, P = 0.062); however, after multivariable adjustment, similar OSs were observed in those patients (HR, 1.219; 95% CI, 0.91–1.633; P = 0.183). After propensity-score matching with a total of 1,560 patients, no significant difference was identified (P = 0.183). A slightly worse OS was seen for stage III RCC patients (HR, 1.561; 95% CI, 0.967–2.522; P = 0.068) than for stage III LCC patients. The 5-year OSs for patients with stage III RCC and stage III LCC were 85.5% and 90.5%, respectively (P = 0.133).
Conclusion
Although the results are inconclusive, tumor location tended to be associated with OS in CC patients with lymph node metastasis, but it was not related to oncologic outcome.
INTRODUCTION
In colon cancer, the current clinicopathological risk factors guiding the prescription of postsurgical therapy for avoiding disease progression and death include the presence of perforation and/or obstruction, vascular invasion, pT4 classification, and tumor stage and grade [1-4]. Because several current gold standard prognostic factors exist, the importance of sidedness as a prognostic factor for patients with colon cancer has received little attention. However, a role has recently been suggested for the primary tumor location as a prognostic factor in patients diagnosed with colon cancer [5]. Several differences in terms of clinical manifestations, pathologic features, and embryologic development exist between right-sided colon cancer (RCC) and left-sided colon cancer (LCC) [1, 6-12]. Furthermore, large genetic database analyses of colon cancers originating in the right and the left colon have demonstrated differential molecular biological tumor patterns between RCC and LCC [6, 7, 10, 11, 13, 14].
According to the current major guidelines, when selecting patients at disease stages II and III for adjuvant chemotherapy, all clinicopathological risk factors, including pT4 classification, vascular invasion, grade, stage, and obstructing and/or perforating presentation, should be considered [1, 15-17]. However, in addition to these standard clinicopathological risk factors, the location of the primary tumor influences the survival benefit of adjuvant chemotherapy for the treatment of colorectal cancer [18]. More aggressive treatments seem to be required to treat RCC patients [1], owing to the prognoses for those patients being inferior to the prognoses for LCC patients. However, the associations of tumor side with oncologic outcomes and its effect on treatment are not clear. Thus, we evaluate the prognostic value of the primary tumor’s location on oncologic outcomes for patients with colon cancer. In our propensity-score matched analysis, we evaluated the prognostic value of primary tumor location for patients with colon cancer.
METHODS
Patients
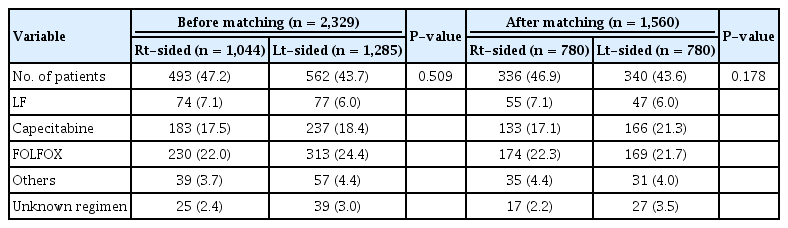
A total of 2,329 patients who underwent curative surgery for colon cancer between January 2009 and December 2012 were included. Of these, 1,285 patients (55.2%) had LCC while 1,044 patients (44.8%) had RCC. RCCs were defined as those arising from the cecum to, and including, the transverse colon. LCCs were defined as those arising from the splenic flexure down to, and including, the rectosigmoid junction. The exclusion criteria were as follows: concurrent distant metastasis at diagnosis, concurrent inflammatory bowel disease, hereditary colorectal cancer syndromes, concurrent malignancy, and prior history of malignancy. All the patients who had colon cancer underwent surgery by seven specialized colorectal surgeons. The study protocol was approved by the Institutional Review Board of Asan Medical Center (registration number: S2017-2357-0001), in accordance with the Declaration of Helsinki. Informed consent form was waived due to the retrospective nature of this study, and approved by the IRB.
Statistical analysis
Survival curves were constructed using the Kaplan-Meier method and were compared using log-rank tests. The associations between clinical factors and RFS were assessed using the Cox proportional hazard regression model. P < 0.05 was considered statistically significant. All statistical analyses were performed using R version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria) and IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA).
Propensity scores were generated by using the R package ‘‘Match It’’ (The Comprehensive R Archive Network: http://cran.r-project.org). Propensity scores to determine matched pairs between the groups were created using eight variables (age, sex, T/N stage, histologic grade, presence of lymphovascular invasion, presence of perineural invasion, and microsatellite instability [MSI] status) that could potentially influence the oncologic outcomes for patients with colon cancer. Group comparisons between before and after matching were performed using the chi-square test or Fisher exact test for categorical variables and the Student t-test for continuous variables.
RESULTS
Patient characteristics
For the present investigation, 2,329 patients diagnosed with colon cancer between 2009 and 2012 were included. The mean follow-up duration was 66.5 ± 16.2 months, and the median follow-up duration was 66 months (interquartile range, 6–94 months). Table 1 compares the patients’ characteristics between the 2 groups; the analysis indicated relevant imbalances for the characteristics of all patients. Patients with RCC had more advanced tumor stages and higher T-stages, more often had mucinous carcinomas, more often had high MSI, were older, and were more often female (all, P < 0.001). The details of adjuvant chemotherapy are summarized in Table 2. No significant differences in the chemotherapy regimens between the 2 groups were fund (before matching: P = 0.509, after matching: P = 0.178).
Oncologic and survival outcomes according to the location of the primary tumor
The overall survival (OS) and recurrence-free survival (RFS) did not differ between RCC and LCC (Table 3). Both systemic and local recurrences were also similar between the 2 groups when analyzed according to stage. Specifically, the 5-year RFS rate for patients with RCC was 89.7%, compared with 88.4% for patients with LCC (P = 0.83). The observed 5-year OS rate for patients with RCC was 90.9% compared with 93.4% for patients with LCC. In the unadjusted analysis, RCC was associated with a marginally significantly increased risk in overall mortality (hazard ratio [HR] of death, 1.297; 95% confidence interval [CI], 0.987–1.704; P = 0.062) (Table 3, Fig. 1A). After multivariable adjustment, patients with RCC had an OS similar to that (HR, 1.219; 95% CI, 0.91–1.633; P = 0.183) in patients with LCC (Table 3). No significant differences in OS and RFS between RCC and LCC were found for patients with stage I or II disease (Table 3).

Overall survival and recurrence according to stage as determined using the Cox proportional hazards model
Adjustment for patients’ characteristics with propensity score matching
After propensity-score matching, a total of 1,560 patients (right-sided, 780; left-sided, 780) were selected for analysis. The baseline characteristics according to the surgical procedures for all patients and for the propensity-matched groups are depicted in Table 1. Significant differences were found in stage, histologic grade, presence of lymphovascular invasion, presence of perineural invasion, and MSI status between the right-sided and the left-sided groups before propensity matching. After matching, no significant differences were observed. In this cohort, for stage III cancer, the OS rate for patients with RCC tended to be worse than that for patients with LCC (HR, 1.561; 95% CI, 0.967–2.522; P = 0.068) (Table 3, Fig. 2D). The 5-year OS rate for stage III patients with RCC was 85.5% compared with 90.5% for stage III patients with LCC. Most of the patients with stage I disease did not receive chemotherapy (1 of 352, 0.3%) whereas most of the patients with stage III disease did (493 of 513, 95.7%). About 52% of stage II patients received adjuvant chemotherapy (360 of 693). The OS for patients with stage II RCC who received adjuvant chemotherapy was similar to that for patients with stage II LCC (91.6% vs. 95.0%, P = 0.093).
DISCUSSION
The results from our study, through a population-based propensity-score adjusted analysis, demonstrate that the prognosis for patients with stage III right-sided colon cancer with lymph node metastasis showed a survival rate inferior to that for patients with stage III left-sided colon cancer. Interest in the potential prognostic effect of primary tumor sidedness peaked after the publication of a prospective study based on patients with systemic metastasis [19]. As a consequence, studies focusing on the prognostic value of primary tumor location have been increasingly performed for patients with metastatic colorectal cancer. As the present study focused on patients who underwent curative resection, evaluating the influence of tumor location was more feasible than in previous studies targeting patients with metastatic colorectal cancer, especially considering that the prognosis in the latter patients can easily be affected by the use of various chemotherapy regimens and targeted agents due to the varying mutational statuses in patients with metastatic colorectal cancer, such as KRAS, NRAS, and BRAF mutations, although a subgroup analysis was performed according to mutational status [20-22]. Furthermore, we independently evaluated the prognostic value of primary tumor location by performing a propensity-matched analysis in the present study.
Several previous studies have found a prognostic impact of tumor location in patients with colorectal cancer. By using the Surveillance, Epidemiology, and End Results (SEER) Program database, Meguid et al. [7] analyzed 77,987 patients with colon cancer. Both their study and the present study used propensity-score matching to evaluate multivariate predictors of outcomes, such as the patients’ age, sex, tumor stage, and grade. In the study of Meguid et al. [7], when the authors controlled for histological grade, tumor stage, and tumor size, patients with RCC showed poorer prognoses than those with LCC. Another study of SEER data by Weiss et al. [23] assessed 53,801 patients with stage I–III primary colon adenocarcinomas. Among their subgroup of stage I or II cancers, no significant difference in the adjusted survival rates was revealed between patients with RCC and those with LCC. However, patients with RCC stage III exhibited an increased mortality rate compared to patients with LCC stage III. Accordingly, the influence of tumor sidedness on survival was concluded to be smaller than the influence of tumor biology, including MSI. Furthermore, 17,641 patients with colorectal cancer were evaluated using a proportional hazard model by Benedix et al. [8]. Even though right-sided primary tumor location was found to be associated with a poorer mean survival and higher mortality risk compared with left-sided primary tumor location, survival was related less to tumor location than to other factors such as age and tumor stage. Lastly, a recent systemic review and meta-analysis performed by Yahagi et al. [6] demonstrated that patients with RCC showed a marginally worse OS than those with LCC. However, only colon cancer patients from Western countries presented a significantly different prognosis according to the primary tumor location in their subgroup analysis whereas those from Eastern countries did not. Similarly, Liu et al. [5] reported that tumor location was not a prognostic factor among Chinese patients. As a result, a suggestion was made that ethnicity might also be as much a prognostic factor as tumor location.
Studies regarding the cause for the influence of tumor location on the prognoses for patients with colorectal cancer have been reported. The effects of both distinguishing genetic profiles and comparing the sensitivities of treatments were reviewed by Shen et al. [18] in order to explain the differences between RCC and LCC. Although significant variations on the molecular level between RCC and LCC were presented, similar survival rates between patients with stage I–III RCC and stage I–III LCC were revealed. This might be due to the development of adjuvant chemotherapies for the treatments of patients with colon cancer. In addition, Moritani and coworkers [24] studied 820 patients with stage I–III colon cancer who underwent radical surgery with curative intention over a 17-year period at a single institution in order to determine the prognostic difference between tumor locations. They used the RFS rate as the primary endpoint rather than the OS rate because the RFS rate explains the metastatic potential, as well as the recurrence patterns, based on the primary tumor location. Patients with RCC stage II or III disease showed a significantly lower RFS rate than those with LCC stage II or III disease, but the results were not consistent for patients with stage I disease. Moreover, distributions of the first recurrence sites were found not to be significantly different between RCC and LCC.
In the present study, only patients with stage III disease showed a difference in OS, but not RFS, according to the tumor location, with borderline significance. The recurrence rate and pattern were not associated with tumor location. These results might reflect that tumor location is not a factor related to oncologic outcomes. The other clinicopathological characteristics, which were already known to be prognostic factors for oncologic outcomes, may have minimized the impact of tumor location. The present study focused on evaluating tumor location as a potential prognostic factor. The main rationale for finding novel prognostic factors is to aid in the decision-making regarding the most beneficial treatment for patients with colon cancer [25]. Whether our results will directly affect the treatment choice is not clear because decision-making is difficult unless the prognostic factor directly relates to the recurrence rate.
Because our results are associated with the survival rate, further studies based on oncologic results from large cohort analyses are required. In particular, because the finding that the tumor location in patients with lymph node metastasis influenced the survival rate, the possibilities of influence on the currently used therapeutics, including chemotherapeutic agents, need to be considered. Furthermore, assessing prognostic differences according to different molecular biology characteristics was not feasible in this study; however, because mutations in biomarkers (KRAS, NRAS, and BRAF) were partly detected in our patients, this needs to be considered in future studies.
Our study has several limitations that must be addressed. First, this study was designed as a retrospective, single-center study although a propensity-score matched analysis was used to mitigate this limitation. Second, this study did not include stage IV patients, for whom the effects of genetic characteristics on treatment can usually be evaluated. Lastly, the assessment of specific biomarkers was insufficiently performed in this study, although distinct molecular biological tumor patterns are widely known to exist. However, the strength of our study is that we demonstrated the value of primary colon cancer location (whether right-sided or left-sided) as a potential prognostic factor in the surgical curative setting and in the presence of lymph node metastasis.
In conclusion, although the results of this study are inconclusive, tumor location tended to be associated with OS in colon cancer patients with lymph node metastasis, but it was not related to the oncologic outcome. Therefore, the association between tumor sidedness and oncologic outcome needs to be reassessed through an additional study with a larger cohort. Moreover, further studies based on molecular biology analyses are needed to determine any differences according to the location of the primary tumor site.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.