- Search
| Ann Coloproctol > Volume 39(3); 2023 > Article |
|
Abstract
Purpose
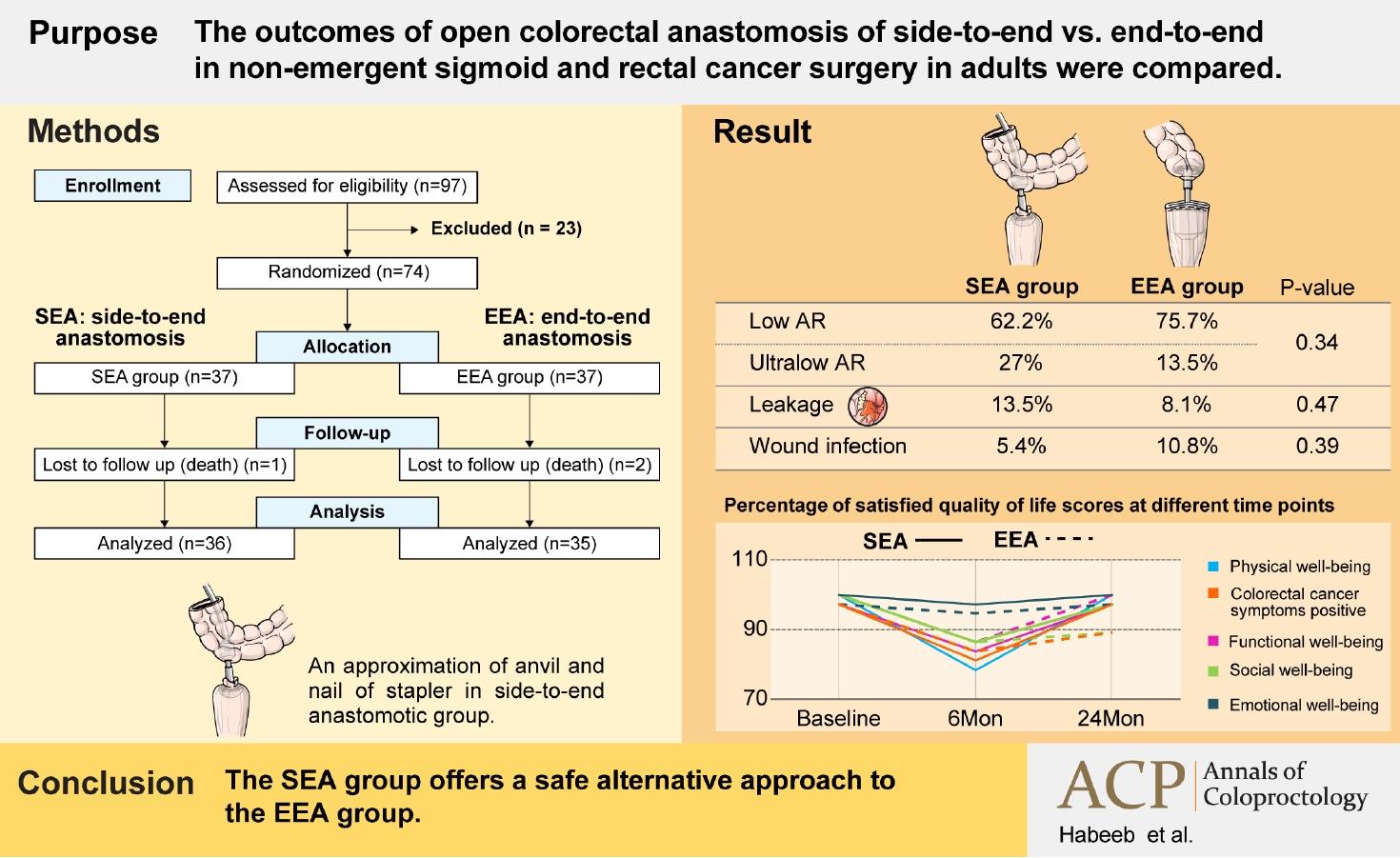
The outcomes of open colorectal anastomosis of side-to-end versus end-to-end in nonemergent sigmoid and rectal cancer surgery in adults were compared.
Methods
A randomized controlled trial on individuals with sigmoid and rectal cancers was conducted between September 2016 and September 2018.
Results
The mean age was 62.58±12.3 years in the side-to-end anastomotic (SEA) group and 61.03±13.98 years in the end-to-end anastomotic (EEA) group. Except for the operative time, intraoperative data revealed no significant differences between the studied groups, and the SEA group revealed that the mean anastomotic time was significantly shorter. Perioperative blood loss, length of stay, reoperation, inpatient death, infection, and bleeding were significantly associated with leakage. There is a statistically significant change regarding the range of bowel frequency in the EEA group only (P=0.04). There is a statistically significant difference regarding incontinence for flatus in the SEA group only (P≤0.001). A statistically significant change in both groups regards incontinence for liquid stools (P≤0.001) and clustering of stools (P≤0.001 and P=0.043). The quality of life in the SEA group significantly dropped at 6 months and then returned to baseline as regards to physical well-being (PWB), functional well-being (FWB), and colorectal cancer symptoms (CCS) with no difference as regards SWB and EWB, while in the EEA group, the exact change happened only as regard PWB and FWB, but SWB and CCS percentage did not return to baseline.
Graphical Abstract
Colon and rectal cancer prevalence come next to bronchogenic and prostatic malignant neoplasms in males, but it is second in females, following cancer of the breast [1]. Despite the fact that 1,000,000 patients are diagnosed each year and thousands die as a result of this cancer, the number of people living with it has increased in recent years as a result of both expensive research tools and novel intervention techniques. Over 2/3 of colorectal cancer patients survived 5 years [2, 3].
The goal of colorectal surgery is to combine well-balanced free margin resection with bowel, urinary, and potency activity [4, 5]. In recent surgery, laparoscopic colorectal surgery for cancer had a well-known corner that challenged the open technique. Nonetheless, due to expense or a lack of qualified laparoscopic colorectal surgeons, the laparoscopic method may not be used in some areas [6]. Colorectal surgery involves a lot of complications. Anastomotic leaking is the most serious complication. Following colon resection and anastomosis, anastomotic leakage can account for up to 15% of cases, resulting in substantial morbidity and death [7–9]. The stapler’s introduction into colorectal surgery allowed for a more homogeneous anastomosis, less pressure on the suture, faster surgery, no intraabdominal contamination, no incursion of anastomotic site perfusion, and a lower prevalence of anastomotic leakage [10–12]. Low anterior resection syndrome (LARS) is correlated with rectum resection and affects quality of life (QoL) [13, 14].
During colorectal cancer surgery, there is no definitive approach of resection and anastomosis, and surgeons usually follow their experience. We conducted a randomized clinical trial to compare the outcomes of 2 different open techniques for resection and anastomosis employing a single circular stapler. The main goal was to compare the incidences of anastomotic leakage after both techniques. The secondary goal was to compare both groups’ functional outcomes and QoL. The tertiary goal was to assess the rate of morbidity and mortality in both techniques.
The study was approved by the Ethical Committee of Faculty of Medicine, Zagazig University (No. HWD134) and performed in accordance with the principles of the Declaration of Helsinki. The work has been reported in line with CONSORT (Consolidated Standards of Reporting Trials) guidelines. After the study was included, all involved persons gave their informed written consent for participation and publication.
From September 2016 to September 2018, a prospective randomized trial was conducted at the colorectal surgery unit of Zagazig University Hospital. Because the frequency of leakage in the published study [15] was 29.2% versus 5.4%, the sample size for each group will be 37, with a power of 80% and a confidence level of 95% appropriate for statistical significance (P< 0.05). It is a simple random sample with a balance. A random sequence computer was used to distribute patients at random. Patients were assigned numbers at random in sealed envelopes that were unsealed immediately before the anastomosis was completed intraoperatively. Until the end of the trial, the patients had no idea which group they were in.
In order to participate in the study, patients had to fulfill the following criteria: sigmoid and rectum adenocarcinoma confirmed by biopsy and histopathological examination, R0 resection with immediate restoration of colorectal continuity with or without covering proximal ostomy, patients aged > 8 years in both sexes, with or without neoadjuvant treatment, residual rectal stump of > 3 cm from the anal verge for carcinoma rectum, and clinically normal function of the anal sphincter. Exclusion criteria included patients younger than 18 years, psychopathic patients, recurrent or nonresectable cancer, combination operations, and complicated cancer (e.g., obstructed or perforated tumor-infiltrating anal sphincter), and previous left-sided colorectal surgery or anorectal surgeries. After the exclusion, a total of 74 patients diagnosed with sigmoid cancer and rectal cancer underwent colorectal cancer surgery from September 2016 to September 2018. A flowchart of the inclusion and exclusion criteria is shown in Fig. 1.
The incidence of anastomotic leak during hospital admission and the first 30 days after surgery is the primary outcome. Intestinal function and QoL 1, 6, 12, and 24 months after surgery are secondary outcomes. The operative time (minute), intraoperative hemorrhage (mL), vessel injury at operation, hematoma/seroma, the span of hospital admission (day), wound infection at any time point, in-hospital mortality, intensive care unit/days (intensive care unit admission), and stricture are the tertiary outcomes (within 90 days postoperative).
The International Study Group of Rectal Cancer (ISREC) depicted an anastomotic leak and designated grades A, B, and C for an ordered approach for dealing with anastomotic leak following colorectal surgery [16]. The Low Anterior Resection Syndrome Questionnaire, which consists of 5 questions, assesses intestinal function (gas incontinence, incontinence of liquid stool, frequency of bowel movements, clustering of stools, and urgency). Functional Assessment of Cancer Therapy-Colorectal (FACT-C) measures QoL. The FACT-C is made up of physical well-being (PWB), social well-being (SWB), emotional well-being (EWB), functional well-being (FWB), and colorectal cancer symptoms (CCS) [17]. Postoperative morbidity was assessed by the ClavienDindo classification [18]. Anterior resection is classified as high (> 10 cm), low (> 5, ≤ 10 cm), or ultralow (within 3–5 cm) based on colonoscopy measurements of tumor location from the anal verge [19].
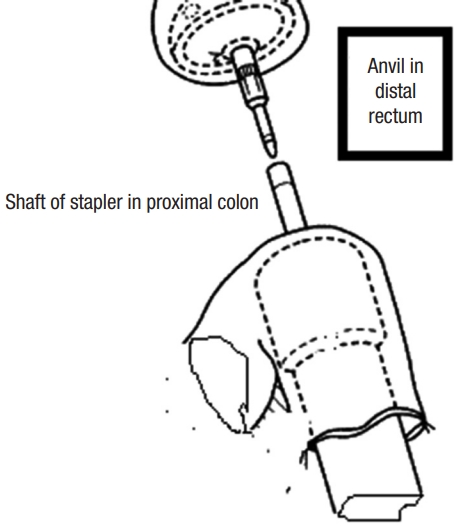
Cancer staging, including total colo-proctoscopy with tumor sample, computed tomography, and magnetic resonance imaging of the abdomen, pelvis, and chest, were all part of the preoperative preparation. In rectal cancers, our center employs neoadjuvant chemoradiotherapy. Preparation of the bowels by oral laxatives (e.g., Minalax tablets, 2 tablets 24 hours and 12 hours before surgery). Metronidazole 500 mg and ceftriaxone 1 g are given at the start of anesthesia. Intravenously administered. Low molecular weight heparin (4,000 IU) is used to avoid deep venous thrombosis. General anaesthesia with cuffed endotracheal intubation in all cases. For 24 months, all surgeries were performed in a single center in the colorectal surgery unit. At least 1 of the 4 senior surgeons were present to ensure that the criteria for inclusion were met. Randomization was done when cancer resection became acclimated to having macroscopic free margins. The Lloyd-Davies position was used on the patient. An incision was made in the lower midline. All patients underwent a standardized anterior resection by dedicated, specialized colon and rectal surgeons. The left colon was mobilized to a position proximal to the splenic flexure. The inferior mesenteric artery was ligated proximal to the left colic artery. The rectum was dissected down to the anorectal junction at the pelvic floor, with total clearance of the mesorectum. The rectum was removed with at least 2 cm of distal tumor clearance. The clamp was replaced after the rectum stump was cleansed with povidone-iodine. In sigmoid cancer, the sigmoid colon was excised with a 10-cm safety margin above the tumor and the rectum as the lower border. The anvil was introduced inside the rectal stump and secured in place with purse-string stitches using polypropylene 2-0 in an antegrade technique. A nail was driven 4 to 6 cm from the colonic opening stump through the end of the opened colon and the colonic wall. The anvil and nail were approximated together (Fig. 2). In the retrograde technique, the anvil head was placed in the proximal colon and the purse-string suture was secured with polypropylene 2-0. After careful anal dilatation, the circular stapler’s shaft was inserted into the anal canal. The stapler was tightened, shot, and then removed smoothly. In the antegrade group, the colonic stump was closed in 2 layers (the inner layer by absorbable vicryl 2/0 suture and the outer layer by nonabsorbable silk 2/0 suture). To assess the anastomosis integrity, continuous circular 2 doughnuts and air leakage testing are used. The pelvic cavity was filled with physiological saline air supplied through the anus by syringe after a clamp was placed on the proximal colon. In most cases, covering ileostomy was done in the middle and lower rectal anastomosis. In any other cases with questionable anastomosis, a covering ileostomy was performed and closed 4 to 6 weeks later, based on the intraoperative surgeon’s assessment. Two drains were placed, one intraperitoneal and the other near the anastomotic line in the pelvis. Fluid was allowed as long as it was tolerated. Antibiotics were given for 5 days, and anticoagulants were given until the patients were completely cured. When a drain contained less than 50 mL for 3 days, it was removed. One month and 6, 12, 24 months after returning home is our center protocol for the follow-up phase (2 years) and it depends on patient capability. Patients were reunited by mail, phone, and at the outpatient clinic or whenever there was complains from the patients. Following up on the patient included a complete medical history and physical examination to rule out any potential complications, a colonoscopy after 6 months to check for stricture at the anastomosis site, and a QoL and intestinal function questionnaire to assess QoL and function. LARS and QoL surveys were required of all patients. Three patients lost during the follow-up period and 3 patients died after the first-month assessment and before the 6-month evaluation (1 in SEA group and 2 in EEA group).
Data from the history, physical exam, lab tests, and outcome measures were recorded, entered, and analyzed using Microsoft Excel (Microsoft Corp). Data was then imported into IBM SPSS ver. 20.0 (IBM Corp) for analysis. Depending on the type of data (qualitative as number and percentage, quantitative as mean± standard deviation), the following tests were performed to determine significance. A chi-square test is used to determine the difference in qualitative variables (× 2) multiple by analysis of variance or Fredman, independent predictors by logistic regression. Results were classified as significant if P-values were set at < 0.05 or < 0.001.
There were no statistically significant differences between the studied groups as regards to demographic and preoperative variables. The mean age was 62.58±12.30 and 61.03±13.98 years in SEA and EEA groups, respectively. Most cases were male patients (38 of 74). The most common comorbidities included hypertension (17.5% of patients in both groups) and diabetes mellitus (16.2%). Most of the tumor sites are located in the upper rectum (69.0%), followed by the middle and lower rectum (20.0%), and the least number is in the sigmoid (11.0%) (Table 1).
No statistically significant difference was found between the studied groups regarding all intraoperative variables except operative time. The mean anastomotic time of SEA group was highly statistically significantly shorter (P< 0.001). Based on the surgical procedures, 8 cases of high anterior resection (10.8%; 4 in SEA group and 4 in EEA group), 41 cases of low anterior resections (23 in SEA group and 28 in EEA group), and 15 cases of ultralow resections (10 in SEA group and 5 in EEA group) were performed (P= 0.34). The overall incidence of intraoperatively diagnosed air leakage was 6.8% (5 of 74 patients). Air leakage after SEA was 5.4%, while EEA was 8.1% (P= 0.64). The most frequently used stapler’s caliber was 33 mm in 43 cases (58%) (P= 0.77) (Table 2).
No statistically significant difference was found between the studied groups as regards all items of postoperative variables. There were 2 cases (5.4%) of anastomotic line bleeding in SEA group and 1 (2.7%) in EEA group. There were 4 cases of stricture of the anastomosis in both groups (2.7% and 8.1%). Postsurgical complications were surgical site infection (5.4% and 4.0%), ileus (2.7% and 5.4%), adhesive intestinal obstruction (2.7% and 5.4%), and intraabdominal abscess (13.5% and 8.1%). Anastomotic leakage occurred in 5 patients (13.5%) in the antegrade group and 3 patients (8.1%) in the retrograde group (P = 0.47). Most cases were grade C leakage (10.8% and 2.7%). The majority of both groups were in class III, followed by classes II and I (Table 3).
Univariate analysis of anastomotic leak showed that perioperative blood loss (P= 0.001), length of stay (P= 0.002), reoperation (P= 0.01), inpatient death (P< 0.001), infection (P< 0.001) and bleeding (P = 0.018) were significantly associated with leakage (Table 4).
Clinical LARS score results comparing SEA and EEA showed a statistically significant change regarding the range of bowel frequency in the EEA group only (P= 0.04). There is a statistically significant difference regarding incontinence for flatus in the SEA group (P< 0.001). There was a statistically significant change in both groups regarding incontinence for liquid stools (P < 0.001) and clustering of stools (P< 0.001 and P= 0.043) (Table 5).
The percentage of satisfied QoL scores at different time-points showed that the QoL in the antegrade group significantly dropped at 6 months and returned to baseline after that as regards PWB, FWB, and CCS with no difference as regard SWB and EWB, while in the retrograde group, the exact change happened only as regard PWB and FWB, but SWB and CCS percentage did not return to baseline (Table 6).
In this study, we evaluated 2 alternative colorectal anastomosis procedures following open colorectal cancer resection: SEA (antegrade approach) and EEA (retrograde approach). Gas incontinence is statistically significant in the SEA group; operative time and anastomotic time were shorter in the SEA group. The postoperative anastomotic leak was not significantly different between both groups. Postoperative LARS and QoL are better in the SEA group than in the EEA group. The SEA group is a safe alternative to the EEA group.
Postoperative anastomotic leakage has been found to range between 1.8% and 24% in earlier studies, with substantial morbidity and death [20–23]. In this study, the SEA group had a higher incidence and severity of anastomotic leakage than the EEA group, but the difference was statistically insignificant. A higher incidence of leakage in SEA group may be attributed to anastomotic site bleeding and postoperative pelvic abscess. Anastomotic site bleeding means insecure anastomosis and pelvic abscess hinders anastomotic healing. These 2 factors were the causes of the higher incidence of anastomotic leaks in the SEA group. Although previous studies stated that anastomotic leaks are caused by a variety of factors, including sex, tumor site, body mass index, anastomotic method, unsuitability of purse-string suture, anastomotic site contamination, and mucosal tears [24–31], we disagree with prior studies since there is no significant difference between the analyzed groups in terms of age, sex, or anastomosis site, and univariate analysis did not indicate a link between anastomosis site and anastomotic leak. Our results also stated previously unknown risk factors for leakage, including perioperative blood loss, reoperation, wound infection, and bleeding from the anastomotic line. The incidence of anastomotic leak is usually higher in lower anastomosis; in our study, however, the covering ileostomy decreased this incidence, and no occurrence of leakage after the ileostomy was closed. Splenic injury has a greater morbidity rate, and it can be avoided by exposing the area properly and avoiding tugging on the splenocolic ligament [32]. Accidental spleen injury is higher in the EEA group than in the SEA group, but the difference is not statistically significant. Splenic damage is more common in the EEA group, owing to a higher number of cases undergoing splenic flexure mobilization than in SEA group. According to a univariate study, perioperative blood loss is substantially related to anastomotic leakage. We discovered that 2 of the patients who had splenic damage also had anastomotic leakage. Splenic damage occurred in 1 patient in each group who experienced postoperative anastomotic leakage.
Different attempts have been postulated to detect intraoperative anastomotic leak. According to Ricciardi et al. [33] and Beard et al. [34], air leak testing has been shown to be beneficial in detecting intraoperative leakage following colorectal resection and anastomosis. They claimed that this test might detect the leak in 7.9% to 25% of cases [33, 34]. In the current study, air leakage was detected intraoperatively in 5 of 74 patients (6.8%, 2 in SEA group, 3 in EEA group) using an air leakage test that we found to be easy and rapid, and we believe it is wise to employ this test in all cases. Re-anastomosis was required in 3 cases. The air leak in the EEA group was most likely caused by the difficult entry of the stapler shaft into the anal canal, which resulted in mucosal and submucosal tears. In terms of intraoperative air leakage, however, there was no statistically significant difference between the 2 groups.
Martellucci [35] declared that up to 80% of patients who underwent colorectal surgery developed LARS. There is a statistically significant change in the range of bowel frequency in the EEA group in the present study. This is due to the loss of most of the rectal reservoir. There is a statistically significant difference regarding incontinence for flatus in the SEA group only due to the accumulation of gas in the colonic pouch that overcomes the anal sphincter. There is a statistically significant change in both groups regarding incontinence for liquid stools and clustering of stools.
A study confirmed the effect of cancer on the QoL of patients who underwent colorectal surgery [36]. In the present study, it is mesmerizing to find that the QoL in the SEA group significantly drops at 6 months and returns to baseline after that while in the EEA group, the exact change occurred but did not return to baseline. This is attributed to the fact that the colonic pouch acts as a neorectum with improving LARS.
In our research, we discovered that fashioning the anastomosis in the SEA group takes less time than in the EEA group (131.2 minutes vs. 23.513 minutes) and that the operative time in SEA group takes less time than in EEA group (15,013 minutes vs. 18,410 minutes). Because of maneuvers in the anal region to enter the stapler, such as anal dilatation and repeated introduction of the stapler in a smooth curve, EEA group takes longer than SEA group.
According to multiple research studies, anastomosis stricture occurs in 8% of cases and is caused by anastomotic site ischemia or anastomotic leakage [37, 38]. In this study, the EEA group had a higher frequency of anastomosis stricture than the SEA group (1 of 37 [2.7%] and 3 of 37 [8.1%]), although leakage was more in the SEA group. Three endoscopic dilatation procedures were necessary for each patient, with 1-month intervals between them. There were no patients who needed surgical reintervention, and no stricture patients complained of leakage. We believe the stricture is caused by ischemia at the anastomosis site as a result of extensive dissection next to the anastomosis with subsequent ischemia and the use of stapler 28 for colorectal anastomosis. A recent study by Back et al. [39] highlighted the need to measure mucosal blood flow in the remaining rectal stump using laser Doppler flowmetry to minimize stricture induced by ischemia of the stump. Local recurrence was not found within this short 24-month follow-up period. Previous studies in the literature, on the other hand, revealed a 3.5% to 12.0% local recurrence [40, 41]. It may be a limitation of this study. The death rate in this study was 2.7% in the SEA group and 5.4% in the EEA group. Two patients died due to grade C anastomotic leaks, 1 in each group. The third patient died of central nervous system complications. A similar study showed a mortality rate of 2.4% [37]. Our study was not without limitations for mono-center study, short follow-up period to evaluate recurrence, small sample size, and a subanalysis between the middle and lower rectum groups were not included. In a conclusion, although gas incontinence is statistically significant in the SEA group, operative time and anastomotic time were shorter in the SEA group. The postoperative anastomotic leak was not significantly different between both groups. Postoperative LARS and QoL are better in the SEA group than in the EEA group. The SEA group is a safe alternative to the EEA group.
Fig. 1.
A flowchart of the inclusion and exclusion of patients in the study. SEA, side-to-end anastomotsis; EEA, end-to-end anastomosis.

Table 1.
Demographic and preoperative data of the studied groups
| Characteristic | SEA group (n = 37) | EEA group (n = 37) | P-value |
|---|---|---|---|
| Sex | 0.85 | ||
| Male | 21 (56.8) | 17 (45.9) | |
| Female | 16 (43.2) | 20 (54.1) | |
| Age (yr) | 62.58 ± 12.30 | 61.03 ± 13.98 | 0.81 |
| < 50 | 8 (21.6) | 11 (29.7) | - |
| 50–70 | 23 (62.2) | 21 (56.8) | 0.72 |
| > 70 | 6 (16.2) | 5 (13.5) | - |
| Body mass index (kg/m2) | 30.53±3.33 | 29.03±3.12 | 0.061 |
| ASA PS classification | |||
| I | 7 (18.9) | 2 (5.4) | - |
| II | 4 (10.8) | 7 (18.9) | 0.08 |
| III | 22 (59.5) | 18 (48.6) | - |
| IV | 4 (10.8) | 10 (27.0) | - |
| Site of tumor from anal vergea (cm) | |||
| Sigmoid ≥ 15 | 4 (10.8) | 4 (10.8) | - |
| Upper rectum ≥ 10–15 | 23 (62.2) | 28 (75.7) | - |
| Middle-low rectum < 10 | 10 (27.0) | 5 (13.5) | 0.34 |
| Tumor stage | |||
| I | 1 (2.7) | 2 (5.4) | - |
| II | 14 (37.8) | 10 (27.0) | 0.67 |
| III | 18 (48.6) | 22 (59.5) | - |
| IV | 4 (10.8) | 3 (8.1) | - |
| Previous abdominal/pelvic surgery | 0.61 | ||
| Yes | 12 (32.4) | 10 (27.0) | |
| No | 25 (67.6) | 27 (73.0) | |
| Magnetic resonance imaging | |||
| T1 | 3 (8.1) | 2 (5.4) | - |
| T2 | 4 (10.8) | 3 (8.1) | 0.083 |
| T3 | 29 (78.4) | 22 (59.5) | - |
| T4 | 1 (2.7) | 10 (27.0) | - |
| N0 | 7 (18.9) | 3 (8.1) | - |
| N1 | 11 (29.7) | 22 (59.5) | 0.061 |
| N2 | 19 (51.4) | 12 (32.4) | - |
| Steroid treatment | 3 (8.1) | 1 (2.7) | 0.63 |
| Serum albumin, > 3.5 g/dL | 37 (100) | 37 (100) | > 0.999 |
| Diabetes mellitus | 5 (13.5) | 7 (18.9) | 0.52 |
| Hypertension | 8 (21.6) | 15 (40.5) | 0.07 |
| Coronary artery disease | 1 (2.7) | 2 (5.4) | 0.55 |
Table 2.
Intraoperative variables in studied groups
| Variable | SEA group (n=37) | EEA group (n=37) | P-value |
|---|---|---|---|
| Surgical procedure | |||
| Sigmoid colectomy (high anterior resection) | 4 (10.8) | 4 (10.8) | - |
| Anterior resection | |||
| Low (upper 2/3 rectal cancer) | 23 (62.2) | 28 (75.7) | 0.34 |
| Ultralow (lower 1/3 rectal cancer) | 10 (27.0) | 5 (13.5) | - |
| Mobilization of the splenic flexure | 0.17 | ||
| Yes (partial, total) | 30 (81.1) | 34 (91.9) | |
| No | 7 (18.9) | 3 (8.1) | |
| Inferior mesenteric vessels dissection | 0.23 | ||
| Yes | 32 (86.5) | 28 (75.7) | |
| No | 5 (13.5) | 9 (24.3) | |
| Blood loss (mL) | 0.39 | ||
| < 500 | 33 (89.2) | 35 (94.6) | |
| > 500 | 4 (10.8) | 2 (5.4) | |
| Size of stapler (mm) | 0.77 | ||
| 34 | 2 (5.4) | 5 (13.5) | |
| 33 | 23 (62.2) | 20 (54.1) | |
| 31 | 5 (13.5) | 5 (13.5) | |
| 29 | 3 (8.1) | 4 (10.8) | |
| 28 | 4 (10.8) | 3 (8.1) | |
| Operative time (min) | 150.0 ± 13.0 | 184.0 ± 10.0 | < 0.001** |
| Anastomotic time (min) | 13.0 ± 1.2 | 23.5 ± 13.7 | < 0.001** |
| Intraoperative anastomotic line bleeding | 1 (2.7) | 2 (5.4) | 0.55 |
| Intraoperative anastomotic leak | 2 (5.4) | 3 (8.1) | 0.64 |
| Dealing with anastomotic leak | 0.71 | ||
| Re-anastomosis | 1 (2.7) | 2 (5.4) | |
| Suturing | 1 (2.7) | 1 (2.7) | |
| Urinary bladder/ureteric injury | |||
| Absent | 36 (97.3) | 35 (94.6) | 0.55 |
| Present | 1 (2.7) | 2 (5.4) | - |
| Intestinal injury | |||
| Absent | 35 (94.6) | 36 (97.3) | - |
| Present | 2 (5.4) | 1 (2.7) | 0.55 |
| Splenic injury | |||
| Absent | 36 (97.3) | 35 (94.6) | - |
| Present | 1 (2.7) | 2 (5.4) | 0.55 |
| Dealing with splenic injury | |||
| Preservation | 1 (2.7) | 1 (2.7) | 0.55 |
| Splenectomy | 0 (0) | 1 (2.7) | - |
Table 3.
Postoperative parameters and complications
Table 4.
Univariate analysis of anastomotic leak
| Variable | Anastomotic leak (n=8) | No anastomotic leak (n=66) | P-value |
|---|---|---|---|
| Agea (yr) | 53 ± 13 | 61 ± 24 | 0.29 |
| Sex | 0.17 | ||
| Male | 6 (75.0) | 25 (37.8) | |
| Female | 2 (25.0) | 41 (62.2) | |
| ASA PS classification | 0.53 | ||
| I | 3 (37.5) | 41 (62.2) | |
| II | 2 (25.0) | 12 (18.2) | |
| III | 2 (25.0) | 7 (10.6) | |
| IV | 1 (12.5) | 6 (9.0) | |
| Body mass indexb (kg/m2) | 32.53 ± 2.38 | 29.40 ± 1.20 | 0.09 |
| Diagnosis | 0.17 | ||
| Cancer sigmoid | 2 (25.0) | 6 (9.0) | |
| Cancer rectum | 6 (75.0) | 60 (91.0) | |
| Clavien-Dindo classification | 0.11 | ||
| I | 1 (12.5) | 22 (33.3) | |
| II | 1 (12.5) | 19 (28.7) | |
| III | 5 (62.5) | 15 (22.7) | |
| IV | 1 (12.5) | 10 (16.3) | |
| Surgical approach | 0.45 | ||
| Side-to-end approach | 3 (37.5) | 34 (51.5) | |
| End-to-end approach | 5 (62.5) | 32 (48.5) | |
| Surgical procedures | 0.17 | ||
| Sigmoid colectomy | 2 (25.0) | 6 (9.0) | |
| Rectal resection | 6 (75.0) | 60 (91.0) | |
| Perioperative blood loss (mL) | 0.001** | ||
| < 500 | 1 (12.5) | 47 (71.2) | |
| > 500 | 7 (87.5) | 19 (28.8) | |
| Mean days in hospital | |||
| <7 | 2 (25.0) | 51 (77.2) | - |
| ≥7 | 6 (75.0) | 15 (22.8) | 0.002* |
| 90-day Mortality | 3 (37.5) | 0 (0) | < 0.001** |
| Reoperation | 0.01* | ||
| Yes | 4 (50.0) | 9 (13.6) | |
| No | 4 (50.0) | 57 (86.4) | |
| Wound infection | < 0.001** | ||
| Yes | 6 (75.0) | 1 (1.5) | |
| No | 2 (25.0) | 65 (98.5) | |
| Bleeding from anastomosis | 0.018* | ||
| Yes | 2 (25.0) | 1 (1.5) | |
| No | 6 (75.0) | 65 (98.5) |
Table 5.
Clinical low anterior resection syndrome score results comparing SEA and EEA
| Variable | 1 mo | 6 mo | 24 mo | P-value |
|---|---|---|---|---|
| Range of bowel frequency (time/day) | ||||
| SEA | ||||
| > 7 | 2 (5.4) | 0 (0) | 0 (0) | - |
| 4–7 | 10 (27.0) | 6 (16.2) | 2 (5.7) | 0.12 |
| 1–3 | 22 (59.5) | 25 (69.4) | 27 (73.0) | - |
| <1 | 3 (8.1) | 5 (13.5) | 7 (18.9) | - |
| EEA | ||||
| > 7 | 3 (8.1) | 2 (5.4) | 2 (5.4) | - |
| 4–7 | 16 (43.2) | 6 (17.1) | 5 (13.5) | 0.04* |
| 1–3 | 15 (40.5) | 24 (68.5) | 23 (65.7) | - |
| <1 | 3 (8.1) | 3 (8.1) | 5 (13.5) | - |
| P-value | 0.413 | 0.374 | 0.423 | - |
| Urgency of defecation (time/wk) | ||||
| SEA | ||||
| Never | 6 (16.2) | 7 (18.9) | 8 (21.6) | - |
| <1 | 19 (51.4) | 23 (62.2) | 22 (61.1) | 0.29 |
| >1 | 12 (32.4) | 6 (16.7) | 6 (16.2) | - |
| EEA | ||||
| Never | 2 (5.4) | 1 (2.7) | 1 (2.7) | - |
| <1 | 21 (56.8) | 23 (65.7) | 24 (68.5) | 0.56 |
| >1 | 14 (37.8) | 11 (31.4) | 10 (27.0) | - |
| P-value | 0.312 | 0.241 | 0.198 | - |
| Incontinence for flatus (time/wk) | ||||
| SEA | ||||
| Never | 7 (18.9) | 17 (47.2) | 29 (80.5) | - |
| <1 | 21 (56.8) | 15 (40.6) | 4 (10.8) | < 0.001** |
| ≥2 | 9 (24.3) | 4 (10.8) | 3 (8.1) | - |
| EEA | ||||
| Never | 10 (27.0) | 12 (32.4) | 12 (34.2) | - |
| <1 | 9 (24.3) | 13 (37.1) | 14 (37.8) | 0.12 |
| ≥1 | 18 (48.6) | 10 (27.0) | 9 (24.4) | - |
| P-value | 0.015* | 0.16 | < 0.001** | - |
| Incontinence for liquid stools (time/wk) | ||||
| SEA | ||||
| Never | 9 (24.3) | 19 (52.7) | 30 (83.3) | - |
| <1 | 21 (56.8) | 15 (40.6) | 5 (13.5) | < 0.001** |
| ≥1 | 7 (18.9) | 2 (5.4) | 1 (2.7) | - |
| EEA | ||||
| Never | 5 (13.5) | 18 (48.7) | 27 (77.1) | - |
| <1 | 20 (54.1) | 12 (32.4) | 2 (5.4) | < 0.001** |
| ≥1 | 12 (32.4) | 5 (13.5) | 6 (16.2) | - |
| P-value | 0.208 | 0.336 | 0.318 | - |
| Clustering of stool (time/wk) | ||||
| SEA | ||||
| Never | 6 (16.2) | 12 (32.4) | 25 (67.6) | < 0.001** |
| <1 | 17 (45.9) | 16 (43.2) | 5 (13.5) | - |
| >1 | 14 (37.8) | 8 (21.6) | 6 (16.2) | - |
| EEA | ||||
| Never | 3 (8.1) | 7 (18.9) | 10 (27.0) | - |
| <1 | 9 (24.3) | 11 (31.4) | 10 (27.0) | 0.043* |
| >1 | 25 (67.6) | 17 (46.0) | 15 (40.6) | - |
| P-value | 0.037* | 0.061 | 0.002* | - |
Table 6.
Percentage of satisfied quality of life scores at different time-points
| Variable |
SEA group |
EEA group |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6 mo | 24 moa | P-value | Baseline | 6 mo | 24 moa | P-value | |
| Physical well-being | 100.0 | 78.3* | 100.0 | 0.001** | 97.2 | 83.7* | 97.2 | 0.041* |
| Functional well-being | 97.2 | 83.7* | 97.2 | 0.042* | 100.0 | 86.4* | 100.0 | 0.048* |
| Social well-being | 100.0 | 86.4 | 97.2 | 0.098 | 100.0 | 86.4 | 89.1 | 0.189 |
| Emotional well-being | 100.0 | 97.2 | 100.0 | 0.988 | 97.2 | 94.5 | 97.2 | 0.989 |
| Colorectal cancer symptoms positive | 97.2 | 81.1* | 97.2 | 0.035* | 97.2 | 83.7 | 89.1 | 0.199 |
REFERENCES
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108.


2. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol 2019;14:89–103.



3. Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 2007;18:581–92.


4. Chiappa A, Biffi R, Zbar AP, Luca F, Crotti C, Bertani E, et al. Results of treatment of distal rectal carcinoma since the introduction of total mesorectal excision: a single unit experience, 1994-2003. Int J Colorectal Dis 2005;20:221–30.


5. Murphy J, Hammond TM, Knowles CH, Scott SM, Lunniss PJ, Williams NS. Does anastomotic technique influence anorectal function after sphincter-saving rectal cancer resection? A systematic review of evidence from randomized trials. J Am Coll Surg 2007;204:673–80.


6. Hewett PJ, Allardyce RA, Bagshaw PF, Frampton CM, Frizelle FA, Rieger NA, et al. Short-term outcomes of the Australasian randomized clinical study comparing laparoscopic and conventional open surgical treatments for colon cancer: the ALCCaS trial. Ann Surg 2008;248:728–38.

7. Jung SH, Yu CS, Choi PW, Kim DD, Park IJ, Kim HC, et al. Risk factors and oncologic impact of anastomotic leakage after rectal cancer surgery. Dis Colon Rectum 2008;51:902–8.


8. Ho YH. Techniques for restoring bowel continuity and function after rectal cancer surgery. World J Gastroenterol 2006;12:6252–60.



9. Yang L, Huang XE, Zhou JN. Risk assessment on anastomotic leakage after rectal cancer surgery: an analysis of 753 patients. Asian Pac J Cancer Prev 2013;14:4447–53.


10. Korolija D. The current evidence on stapled versus hand-sewn anastomoses in the digestive tract. Minim Invasive Ther Allied Technol 2008;17:151–4.


11. Taflampas P, Christodoulakis M, Tsiftsis DD. Anastomotic leakage after low anterior resection for rectal cancer: facts, obscurity, and fiction. Surg Today 2009;39:183–8.


12. Redmond HP, Austin OM, Clery AP, Deasy JM. Safety of double-stapled anastomosis in low anterior resection. Br J Surg 1993;80:924–7.


13. Bryant CL, Lunniss PJ, Knowles CH, Thaha MA, Chan CL. Anterior resection syndrome. Lancet Oncol 2012;13:e403–8.


14. Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg 2012;255:922–8.

15. Brisinda G, Vanella S, Cadeddu F, Civello IM, Brandara F, Nigro C, et al. End-to-end versus end-to-side stapled anastomoses after anterior resection for rectal cancer. J Surg Oncol 2009;99:75–9.


16. den Dulk M, Noter SL, Hendriks ER, Brouwers MA, van der Vlies CH, Oostenbroek RJ, et al. Improved diagnosis and treatment of anastomotic leakage after colorectal surgery. Eur J Surg Oncol 2009;35:420–6.


17. Ward WL, Hahn EA, Mo F, Hernandez L, Tulsky DS, Cella D. Reliability and validity of the Functional Assessment of Cancer\ Therapy-Colorectal (FACT-C) quality of life instrument. Qual Life Res 1999;8:181–95.

18. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–96.

19. Portier G, Ghouti L, Kirzin S, Guimbaud R, Rives M, Lazorthes F. Oncological outcome of ultra-low coloanal anastomosis with and without intersphincteric resection for low rectal adenocarcinoma. Br J Surg 2007;94:341–5.


20. Nesbakken A, Nygaard K, Lunde OC. Outcome and late functional results after anastomotic leakage following mesorectal excision for rectal cancer. Br J Surg 2001;88:400–4.


21. Bielecki K. Colon anastomosis: a challenge for the surgeon, a risk for the patient. Adv Med Sci 1992;5:233–5.
22. Matthiessen P, Hallböök O, Rutegård J, Simert G, Sjödahl R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg 2007;246:207–14.



23. Kube R, Mroczkowski P, Granowski D, Benedix F, Sahm M, Schmidt U, et al. Anastomotic leakage after colon cancer surgery: a predictor of significant morbidity and hospital mortality, and diminished tumour-free survival. Eur J Surg Oncol 2010;36:120–4.


24. Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg 2011;253:890–9.

25. Platell C, Barwood N, Dorfmann G, Makin G. The incidence of anastomotic leaks in patients undergoing colorectal surgery. Colorectal Dis 2007;9:71–9.


26. Karanjia ND, Corder AP, Bearn P, Heald RJ. Leakage from stapled low anastomosis after total mesorectal excision for carcinoma of the rectum. Br J Surg 1994;81:1224–6.


27. Vallance A, Wexner S, Berho M, Cahill R, Coleman M, Haboubi N, et al. A collaborative review of the current concepts and challenges of anastomotic leaks in colorectal surgery. Colorectal Dis 2017;19:O1–12.


28. Bell S, Kong JC, Wale R, Staples M, Oliva K, Wilkins S, et al. The effect of increasing body mass index on laparoscopic surgery for colon and rectal cancer. Colorectal Dis 2018;20:778–88.


29. 2015 European Society of Coloproctology Collaborating Group. The impact of stapling technique and surgeon specialism on anastomotic failure after right-sided colorectal resection: an international multicentre, prospective audit. Colorectal Dis 2018;20:1028–40.



30. Marecik SJ, Chaudhry V, Pearl R, Park JJ, Prasad LM. Single-stapled double-pursestring anastomosis after anterior resection of the rectum. Am J Surg 2007;193:395–9.


31. Roumen RM, Rahusen FT, Wijnen MH, Croiset van Uchelen FA. “Dog ear” formation after double-stapled low anterior resection as a risk factor for anastomotic disruption. Dis Colon Rectum 2000;43:522–5.


33. Ricciardi R, Roberts PL, Marcello PW, Hall JF, Read TE, Schoetz DJ. Anastomotic leak testing after colorectal resection: what are the data? Arch Surg 2009;144:407–12.


34. Beard JD, Nicholson ML, Sayers RD, Lloyd D, Everson NW. Intraoperative air testing of colorectal anastomoses: a prospective, randomized trial. Br J Surg 1990;77:1095–7.


35. Martellucci J. Low anterior resection syndrome: a treatment algorithm. Dis Colon Rectum 2016;59:79–82.

36. Conroy T, Bleiberg H, Glimelius B. Quality of life in patients with advanced colorectal cancer: what has been learnt? Eur J Cancer 2003;39:287–94.

37. Neutzling CB, Lustosa SA, Proenca IM, da Silva EM, Matos D. Stapled versus handsewn methods for colorectal anastomosis surgery. Cochrane Database Syst Rev 2012;(2):CD003144.

38. Orsay CP, Bass EM, Firfer B, Ramakrishnan V, Abcarian H. Blood flow in colon anastomotic stricture formation. Dis Colon Rectum 1995;38:202–6.


39. Back E, Brännström F, Svensson J, Rutegård J, Matthiessen P, Haapamäki MM, et al. Mucosal blood flow in the remaining rectal stump is more affected by total than partial mesorectal excision in patients undergoing anterior resection: a key to understanding differing rates of anastomotic leakage? Langenbecks Arch Surg 2021;406:1971–7.



- TOOLS
-
METRICS

-
- 0 Crossref
- 1 Scopus
- 2,937 View
- 152 Download
- Related articles in ACP