- Search
| Ann Coloproctol > Volume 38(3); 2022 > Article |
|
Abstract
Fig.┬Ā1.
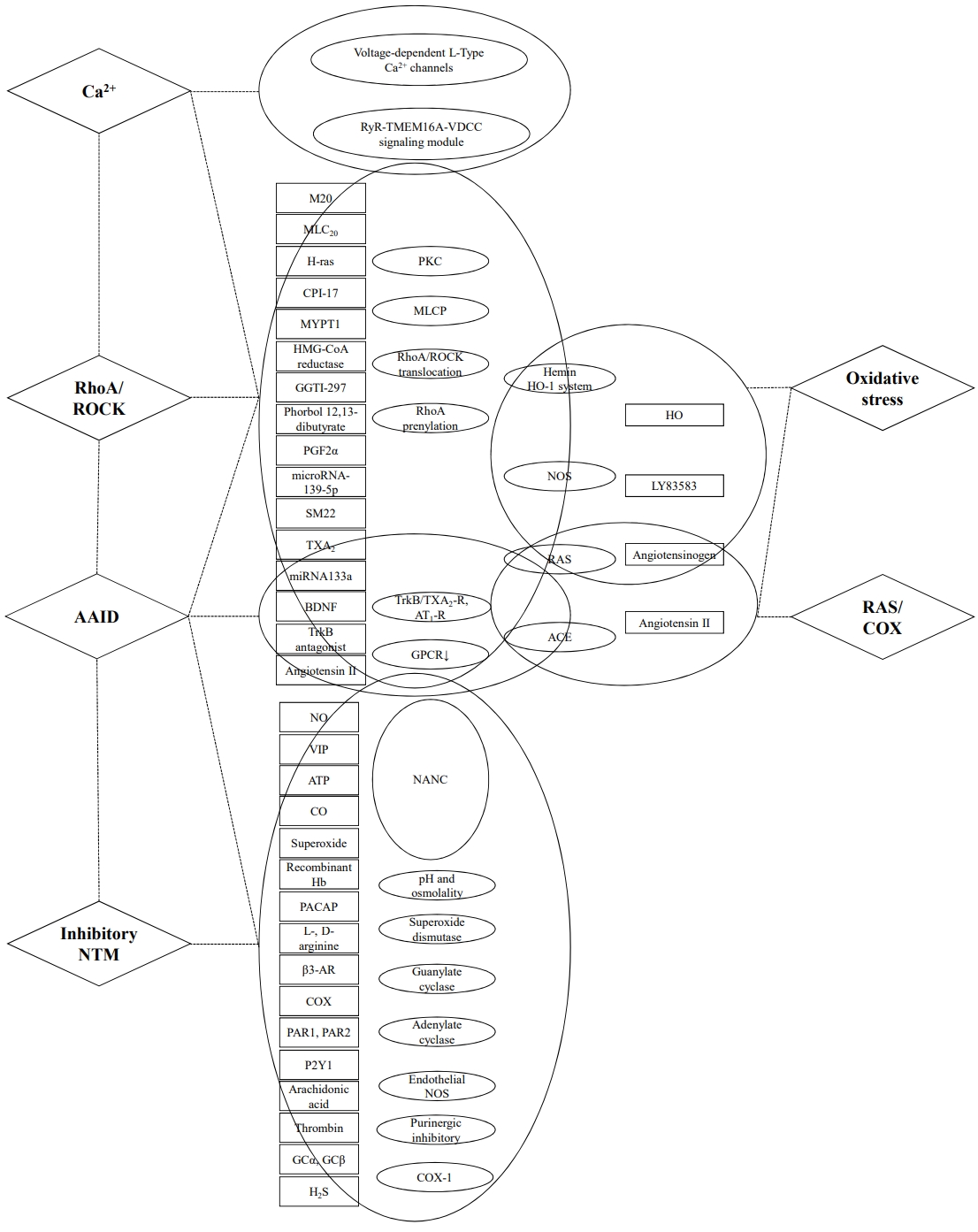
Table┬Ā1.
| Study | Year | IAS harvest | Mediator | Pathway | Action of mechanism | |
|---|---|---|---|---|---|---|
| Calcium (Ca2+) | ||||||
| ŌĆā | Chakder et al. [35] | 1999 | NA | Endothelins 1 and 2 | PKC and the Ca2+-calmodulin pathways | Endothelin-induced contraction of IAS, via inhibition of selective PKC inhibitor H-7 or calmodulin inhibitor W-13 |
| Zhang et al. [34] | 2016 | SM-specific MYPT1, TMEM16A, MLCK knockout mice | Global rise in Ca2+ | RyR-TMEM16A-VDCC signaling module | MLCK activation by a global rise in Ca2+ via a RyR-TMEM16A-VDCC signaling module sets a basal tone of IAS | |
| Cobine et al. [36] | 2020 | SM-GCaMP mice | Spatiotemporal properties of Ca2+ transients | L-type VDCCs | Conduction of CTs rising by slow wave from distal to proximal IAS leading to the maintenance of basal in IAS | |
| Lu et al. [32] | 2021 | SMC-specific TMEM16A deletion mouse | SCaO | RyRŌĆōTMEM16AŌĆōVDCCs pathway | IAS basal tone generated by RyRŌĆōTMEM16AŌĆōVDCCs signaling module mediated by 2 oscillating Ca2+ signals (SCaOs and ACaOs) | |
| Rho/ROCK | ||||||
| Rattan et al. [37] | 2006 | Sprague-Dawley rats | ROCK inhibitor Y-27632 | RhoA/ROCK pathways | Selective ROCK inhibitor (lower doses of Y-27632) relax IAS independent of the NOS/cGMP pathway | |
| Patel et al. [38] | 2007 | Male Sprague-Dawley rats | RhoA-GTP, ROCK II, MLC20, phospho- MYPT1, phospho- MLC20 | RhoA/ROCK pathways | Upregulation of RhoA/ROCK maintains spontaneous tone in IAS | |
| de Godoy et al. [40] | 2007 | H-ras+/ŌłÆ mice | H-ras | Inhibitory RhoA/Rho kinase machinery | H-ras decreases basal tone in IAS via inhibiting RhoA translocation to the plasma membrane, reducing activation of the Rho kinase isoform ROCK II | |
| Patel et al. [41] | 2007 | NA | GGTI-297 | RhoA prenylation blockade (translocation of RhoA to the SMC membrane) | The inhibitory effect of GGTI-297 maintains a basal tone of IAS via decreasing prenylation of RhoA | |
| Rattan [42] | 2010 | Sprague-Dawley rats | HMGCRI | RhoA prenylation leading to RhoA/ ROCK translocation | Relaxation of IAS by HMGCRI simvastatin mediated via decreased downstream of RhoA prenylation and ROCK activity | |
| Singh et al. [43] | 2011 | Human IAS | PDBu | RhoA and ROCK II pathway | PDBu-induced IAS contractility via activation of RhoA/ROCK | |
| Rattan et al. [39] | 2012 | Human IAS | ROCK- and PKC selective inhibitors Y 27632 and G├Č 6850 | RhoA/ROCK pathways | Activation of RhoA/ROCK and downstream signaling determines basal tone in IAS via MLCP inhibition | |
| Rattan et al. [44] | 2015 | Human IAS tissues | Extracellular signal of TXA2, PGF2╬▒ | RAS and arachidonic acid pathways | End products (TXA2, and PGF2╬▒) of both RAS and arachidonic acid pathways causes an increase in the IAS tone via triggering of RhoA/ROCK | |
| Rattan et al. [46] | 2015 | Male Sprague-Dawley rats | SM22 | Actin-binding properties of SM22 interfering with actin-myosin interaction | Phosphorylation of SM22 in ROCK inhibits SM22-actin interaction leads to basal tone as in IAS | |
| Singh et al. [45] | 2017 | Rat | miRNA-139-5p | ROCK2 pathway | Overexpression of miRNA-139-5p causes a decrease in the IAS tone | |
| AAID | ||||||
| Singh et al. [49] | 2016 | Fischer rats (F344 of 6-, 18-, and 26-mo-old age) | miRNA133a | RhoA signaling pathway | Aging-associated miRNA133a and its target gene (RhoA, ROCK2, MYOCD, SRF, and SM22) via regulating RhoA signaling pathway express IAS SM phenotype in the aging | |
| Mohanty et al. [48] | 2019 | Fischer 344 rat | Thromboxane A2/ANG II type | GPCR | Downregulation of GPCR via thromboxane A2 and ANG II type 1 receptors desensitization, lysosomal degradation associated with an aging-related decrease in the basal tone of IAS | |
| Singh et al. [47] | 2020 | Sprague-Dawley rats | BDNF | 1. RhoA/ROCK pathway via TrkB/TXA2-R and AT1-R activation | BDNF-augmented increase in the IAS tone via activation GPCR linked to RhoA/ROCK signaling and NANC Relaxation | |
| 2. NANC relaxation via NO and soluble GC | ||||||
| Singh et al. [33] | 2021 | Male Fischer 344 rats (6-mo-old [young group] and 26-mo-old [old group]) | TrkB antagonist | GPCR-coupled agonist-stimulation by activation of RhoA/ROCK and NANC stimulation | BDNF rescues AAID via RhoA/ROCK and decreases the nitrergic NANC inhibitory neurotransmission. | |
| Oxidative | ||||||
| Krishna et al. [51] | 2014 | Sprague-Dawley rats (20ŌĆō22-wk-old male) | HO-1 | Hemin/HO-1 system | HO (predominantly HO-2 isoform) in neurally mediated relaxation of IAS increases basal tone, and the fibroelastic properties via regulating RhoA/ROCK pathway | |
| Singh et al. [52] | 2014 | Sprague-Dawley rats; 4ŌĆō6 mo (adult) and 24ŌĆō30 mo (aging) | LY83583 | LY83583-mediated a decrease in RhoA/ROCK signal transduction | Oxidative stress is associated with aging-associated decrease in IAS tone via disruption of RhoA/ROCK and downstream signaling cascade | |
| Singh et al. [50] | 2015 | Adult Sprague-Dawley rats | LY-83583 | nNOS inhibition and RhoA/ROCK pathway | Bimodal effect of oxidative stress (lower vs. higher concentra- tion = 0.1 nMŌĆō10 ╬╝M vs. 50ŌĆō100 ╬╝M): lower concentrations leads to an increase in IAS tone via nNOS inhibition and RhoA/ROCK activation by LY-83583 | |
| RAS and COX | ||||||
| De Godoy et al. [53] | 2004 | Male Sprague-Dawley rats | ANG II | ACE | Biosynthesis of Ang II-related peptides by ACE activity modulates basal IAS tone via AT1-R activation | |
| De Godoy et al. [54] | 2005 | Male Sprague-Dawley rats | ANG II precursor angiotensinogen | RAS pathway | RAS regulates basal tone in IAS partially via biosynthesis and releases ANG II by activation of AT1-R | |
| De Godoy et al. [55] | 2006 | Rat | ANG II | Internalization of subtype I receptor(s) (AT1-R) in the plasma membrane and externalization of subtype II receptor(s) (AT2-R) in the cytosol | Translocation of AT1- and AT2-Rs by higher concentrations of ANG II leads to relaxation of the IAS | |
| Inhibitory NTM | ||||||
| Moummi et al. [62] | 1988 | NA | EFS and exogenous VIP | GC and adenylate cyclase | EFS induces relaxation of SM in IAS mediated via guanosine 5'-cyclic monophosphate | |
| Rattan et al. [73] | 1992 | NA | NO | NANC inhibitory pathway | Inhibitory NANC by NO-mediated IAS relaxation | |
| Rattan et al. [74] | 1992 | NA | NO | NANC inhibitory pathway | NO or NO-like substance is an important mediator of IAS relaxation in response to NANC nerve stimulation | |
| Chakder et al. [69] | 1992 | Mice IAS | NO, VIP, superoxide | Superoxide dismutase | IAS relaxation by NO was suppressed by superoxide and reversed by superoxide dismutase | |
| O'Kelly et al. [67] | 1993 | Human IAS tissue | NO | NANC inhibitory pathway | NO-mediate neurogenic relaxation of the human IAS | |
| Rattan et al. [77] | 1995 | Mice IAS | Recombinant hemoglobin | NO pathway | Recombinant hemoglobin suppresses IAS relaxation induced by NO | |
| Rattan et al. [63] | 1997 | NA | PACAP | N-type Ca++-channel blocker Žē-conotoxin | Dual effect: contraction of IAS via the activation of PACAP receptor at P-containing nerve terminals. IAS relaxation by PACAP direct action at nerve terminals of the myenteric inhibitory neurons | |
| Chakder et al. [64] | 1998 | NA | PACAP | NANC inhibitory pathway | PACAP mediated IAS relaxation via the activation of PACAP1/VIP receptor via presynaptic release of PACAP and VIP by NO | |
| Kubota et al. [58] | 1998 | Canine | Transmural field stimulation | Membrane hyperpolarization with relaxation | Membrane hyperpolarization relaxes IAS via EFS in the transitional and upper region of IAS | |
| Banwait et al. [72] | 2003 | Rat | ╬▓3-AR | Endothelial NOS | IAS SM relaxation via partly transduced NOS by ╬▓3-AR activation | |
| Acheson et al. [66] | 2003 | Sheep and human IAS | L-arginine, D-arginine | ph and osmolality | L-arginine independent of NO reduce IAS tone | |
| Jones et al. [68] | 2003 | nNOS knockout mice | NO, antagonists of VIP, ATP, HO | nNOS, nicotinamide adenine dinucleotide phosphate diaphorase HO | NO induces the RAIR primary, and other inhibitory neurotransmitters compensate for the absence of NOS | |
| Rattan et al. [59] | 2005 | wild-type (WT), HO-2 knockout (HO-2ŌłÆ/ŌłÆ) and nNOS knockout (nNOSŌłÆ/ŌłÆ) mice | CO, NO, VIP | NANC inhibitory pathway, and nNOS pathway | Inhibitory NANC mediated via activation of nNOS and partly VIP relax IAS. CO is not associated with inhibitory NANC relaxation, which directly relaxes IAS | |
| McDonnell et al. [60] | 2008 | BALB/c mice | P2Y1 receptors and apamin-sensitive K+ channels | Purinergic inhibitory neural pathway | Membrane hyperpolarization via purinergic transmission relaxes IAS | |
| Koyuncu et al. [78] | 2008 | Rabbit IAS | Isosorbide dinitrate, sodium nitroprusside | NANC inhibitory pathway | NO leads to IAS relaxation via the NANC pathway, but nitrate tolerance was not developed | |
| de Godoy et al. [56] | 2009 | Knockout mice with selective deletion of COX-1 or COX-2 (COX-1ŌĆō/ŌĆō and COX-2ŌĆō/ŌĆō mice) | COX-1 | COX-I pathway | Prostanoids produced via COX-1 provide an external trigger for basal tone in IAS | |
| de Godoy et al. [57] | 2009 | Male Sprague-Dawley rats | Arachidonic acid | COX-I pathway | Arachidonic acid metabolites (PGF2 and thromboxane A2) increases the basal tone of IAS | |
| Acheson et al. [65] | 2009 | Sheep IAS | NO, noradrenaline | NANC inhibitory pathway | Endogenous noradrenaline acts via postjunctional ╬▒1-ARs to antagonize neurogenic relaxations that are largely mediated by NO | |
| Duffy et al. [76] | 2012 | C57BL/6 and W/Wv mice IAS | ATP | Purinergic inhibitory neural pathway | Purinergic hyperpolarization associated relaxation of IAS independent on intramuscular interstitial cells of Cajal | |
| Keef et al. [75] | 2013 | VIPŌłÆ/ŌłÆmice | VIP | NANC inhibitory pathway | Ultraslow relaxation and hyperpolarization mediated by VIP leading to prolonged IAS relaxation | |
| Huang [70] | 2014 | Guinea pig IAS | Thrombin and PAR1 peptide agonists | NO pathway | PAR1 and PAR2 mediate relaxation of IAS | |
| Cobine et al. [71] | 2014 | KitcopGFP/+, C57BL/6 (wild-type), Pdgfr╬▒egfp/+, smMHCCre-egfp, cGKI+/ŌĆō mice | GC (GC╬▒, GC╬▓) and NO | NANC inhibitory pathway (GC-dependent, cGKI independent pathway) | Nitrergic effectors in the PDGFR╬▒-cells induce nitrergic relaxation of IAS mediated by GC within the SIP syncytium | |
| Folasire et al. [61] | 2016 | Porcine IAS | NO, CO, H2S | NANC inhibitory pathway | Simultaneous release of all 3 gaseous transmitters by EFS induces the relaxations of the IAS | |
IASAAID, aging-associated internal anal sphincter dysfunction; ACaO, asynchronized Ca2+ oscillation; ACE, angiotensin-converting enzyme; ANG II, angiotensin II; AR, adrenoceptor; AT1-R, angiotensin II receptor type 1; AT2-R, angiotensin II receptor type 2; BDNF, brain-derived neurotrophic factor; cGKI, cyclic guanosine monophosphate-dependent protein kinase I; cGMP, cyclic guanosine monophosphate; CO, carbon monoxide; COX, cyclooxygenase; CT, Ca2+ transient; EFS, electrical field stimulation; GC, guanylate cyclase; GGTI, geranylgeranyl transferase inhibitor; GPCR, G protein-coupled receptor; GTP, guanosine triphosphate; H2S, hydrogen sulfide; HMGCRI, HMG-CoA reductase inhibition; MLCP, myosin light chain phosphatase; HO, heme oxygenase; IAS, internal anal sphincter; LY-83583, oxidative stress inducer 6-anilino-5,8-quinolinedione; miRNA, microRNA; MLC, myosin light chain; MLCK, myosin light chain kinase; MYPT, myosin phosphatase target subunit; NA, not available; NANC, nonadrenergic noncholinergic; nNOS, neuronal nitric oxide synthase; NO, nitric oxide; NOS, nitric oxide synthase; PACAP, pituitary adenylate cyclase-activating peptide; PAR, proteinase-activated receptor; PDBu, phorbol 12,13-dibutyrate; PDGFR, platelet-derived growth factor receptor; PGF2╬▒, prostaglandin F2╬▒; PKC, protein kinase C; RAIR, rectoanal inhibitory reflex; RAS, renin-angiotensin system; ROCK, Rho-associated protein kinase; RyR, ryanodine receptor; SCaO, synchronized Ca2+ oscillation; SM, smooth muscle; SMC, smooth muscle cell; TMEM16A, transmembrane member 16A; TXA2, thromboxane A2; TXA2-R, thromboxane A2-receptor; TrkB, tyrosine kinase receptor B; VDCC, voltage-dependent Ca2+ channel; VIP, vasoactive intestinal polypeptide.
Table┬Ā2.
| Study | Year | Cell type | Animal (No. of animal) | Type of sphincter injury/confirmation of incontinence | Implantation/factors/scaffolding | Cell tracking | Outcome |
|---|---|---|---|---|---|---|---|
| Inoue et al. [16] | 2018 | ASC sheets | Female Sprague-Dawley rats (n = 18) | Sphincterotomy by the removal of the left semicircle in both the IAS and EAS via a posterior incision/Not confirmed | None | Fluorescence in situ hybridization | Anal manometry, histology |
| Salcedo et al. [17] | 2013 | Mesenchymal stem cell | Age-matched female Sprague-Dawley rats (n = 70) | Incising the IAS and EAS 2ŌĆō3 mm deep+pudendal nerve crush/Confirmed via mi- croscopy | IM or IV injection | Green fluorescent protein | Anal manometry, electromyography, immunofluorescence analysis |
| Salcedo et al. [18] | 2014 | Mesenchymal stem cells | Age-matched female Sprague-Dawley rats (n = 50) | Excision of 25% of the IAS and EAS muscle/Confirmed via microscopy | IM or serial IV injections | Green fluorescent protein | Anal manometry, immunofluorescence, histology |
| Kuismanen et al. [19] | 2018 | hASC | Sprague-Dawley female virgin rats (n = 60) | Acute fourth grade EAS and IAS muscle and mucosa) and sewed back with 6-0 poliglecaprone/Not con- firmed | Polyacrylamide hydrogel carrier, Bulkamid | Micro-computed tomography | Anal manometry, micro-computed tomography imaging, 3D imaging, histology |
| Oh et al. [20] | 2015 | Autologous myoblasts | Male mongrel dogs (19ŌĆō22 kg; 10 wk old) (n = 15) | Resecting 25% of the posterior IAS and EAS by electrocautery/Anal manometry and CMAP confirmed | Polycaprolactone beads | Fluorescent dye PKH-26 | In vitro contractility, CMAP, histology |
| Sarveazad et al. [79] | 2019 | hASC | Male rabbits (n = 7) | Grade 4 tear at EAS and IAS/ Confirmed via histology, percentage of collagen, muscle | Laser (660 nm, 90 sec, immediately after sphincterotomy, daily, 14 days) | Dil solution | Anal manometry, immunofluorescence, histology, collagen analysis, VEGFA, Ki67 mRNA, vimentin mRNA gene expression profiling |
| Hecker et al. [80] | 2005 | SMCs from rabbits IAS | In vitro | None | 3D cylindrical IAS ring/fibrin gel and 5-mm diameter SYLGARD mold | None | In vitro physiologic functionality (generating spontaneous basal tone, kinetics, and dose-response curve of force generated by IAS ring), histology |
| Somara et al. [81] | 2009 | SMCs from human IAS | In vitro | None | 3D bioengineered ring model | None | In vitro physiologic functionality (contractile properties and force generation in response to acetylcholine, PKC inhibitor calphostin- C, Rho/ROCK inhibitor Y-27632, permeable Rho/ROCK inhibitor c3- exoenzyme, and PKC activator PDBu) |
| Raghavan et al. [13] | 2011 | SMCs from mouse IAS | RAG1ŌłÆ/ŌłÆ mice (NA) | None | Implantation of bioengineered IAS construct in back of mice/IM-FEN cells | None | In vivo myogenic and neuronal components (basal tone; spontaneous generation, relaxation, and recovery by VIP-ergic, EFS, cholinergic, KCl-induced), histology |
| Raghavan et al. [83] | 2010 | SMCs from Human and rabbits IAS | C57BL/6J mice | None | Implant subcutaneously on the dorsum of mice/microosmotic pump+fibroblast growth factor-2 | None | In vivo physiologic functionality (generation of spontaneous basal tone; contraction and relaxation by responding to cholinergic, nitrergic, and VIP-ergic stimulation and potassium chloride), histology |
| Raghavan et al. [14] | 2014 | SMCs from human IAS | Rat | None | Implant surgically into the perianal region/innervation of enteric neuronal progenitor cells from the human colorectum | None | In vivo physiologic functionality (relaxation of IAS ring in response to EFS, VIP), immunohistochemical analysisASC, |
ASC, adipose stem cell; CMAP, compound muscle action potential; EAS, external anal sphincter; EFS, electrical field stimulation; hASC, human adipose stem cell; IAS, internal anal sphincter; IM, intramuscular; IM-FEN, immortomouse fetal enteric neuronal; IV, intravenous; mRNA, messenger RNA; NA, not available; PDBu, phorbol 12,13-dibutyrate; PKC, protein kinase C; RAG1, recombination activating gene 1; ROCK, Rho-associated protein kinase; SMC, smooth muscle cell; VEGFA, vascular endothelial growth factor A; VIP, vasoactive intestinal polypeptide; 3D, 3-dimensional.
REFERENCES
-
METRICS

- Related articles in ACP
-
Artificial Bowel Sphincter for Fecal Incontinence.2006 October;22(5)







