Characteristics and Survival of Korean Anal Cancer From the Korea Central Cancer Registry Data
Article information
Abstract
Purpose
In Korea, anal cancer is rare disease entity with specific clinical characteristics. Therefore, no survival analysis with a sufficient patient population has been performed. The aim of this study was to evaluate the characteristics of Korean anal cancer, focusing on the survival according to tumor histologies, sex, and a specific age group, using the nationwide cancer registry.
Methods
Using the Korea Central Cancer Registry, we analyzed a total of 2,552 cases from 1993 to 2010. We assessed the 5-year relative survival by using tumor histology. In addition, survival differences of Surveillance Epidemiology and End Results (SEER) stage were analyzed for both sexes and for young-age cancer (younger than 40 years) and advanced-age cancer (older than 70 years).
Results
The 5-year relative survival among anal cancer patients increased from 38.9% for the period 1993-1995 to 65.6% for the period 2006-2010. The anal squamous cell carcinoma was the most common histology and showed better survival than other types of cancer. Females demonstrated better survival than males in all SEER stages. The 5-year survivals for patients in whom anal cancer developed before the age of 40 and at or after the age of 40 were 62.4% and 51.6%, respectively. The 5-year survival for patients in whom cancer developed at or after the age of 70 was much worse than that for patients in whom the cancer had developed prior to that age.
Conclusion
Korean anal cancer has certain distinctive characteristics of survival according to tumor histology, sex, and age. Despite limitations on available data, this study used the nationwide database to provide important information on the survival of Korean patients with anal cancer.
INTRODUCTION
Anal cancer is a rare disease and accounts for about 0.1% of the total number of cancer cases per year in Korea [1]. Anal cancer may develop more commonly at an advanced age, especially ages older than sixty. Human papillomavirus (HPV) and immunosuppression, including human immunodeficiency virus (HIV) infection, may be associated with the risk of anal cancer [2, 3]. In Korea, the at-risk population, including women with previous cervical HPV-related disease, immune-suppressed transplant recipients, and HIV-positive individuals, has increased. Anal cancer generally consists of various cell types, including squamous cell carcinomas, adenocarcinomas, melanomas, etc. Therefore, the treatment plan and the prognosis for anal cancer differ according to the histologic type. For this clinically-rare and complex disease entity, no detailed study, including a sufficient number of patients, of survival has been performed for anal cancer in the Korean population as a whole.
The Korea Central Cancer Registry (KCCR) was initially launched as a nation-wide hospital-based cancer registry by the Ministry of Health and Welfare in 1980. The KCCR has covered the whole population under the Population-based Regional Cancer Registry program since 1999. Thus, an analysis of this registry can be informative to understand the features of anal cancer in Korea. In addition, such an analysis may avoid an institutional referral bias. The aim of this study was to evaluate the characteristics of Korean anal cancer, focusing on the survival according to a specific age group, sex, or tumor histology, using the KCCR data.
METHODS
Data on the anal cancer patients diagnosed between 1993 and 2010 were obtained from the Korea National Cancer Incidence Database. A total of 2,552 patients were analyzed in this study. The primary cancer was classified according to the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) [4] and converted to the classification system used by the International Classification of Diseases, 10th edition [5]. The histology of anal cancers was classified into four groups: squamous cell carcinoma (ICD-O-3: 8050/3-8076/3, 8083/3-8084/3, 8123/3-8124/3), adenocarcinoma (ICD-O-3: 8140/3-8145/3, 8190/3-8231/3, 8260/3-8263/3, 8310/3, 8401/3, 8480/3-8490/3, 8550/3-8551/3, 8570/3-8574/3, 8576/3), melanoma (ICD-O-3: 8720/3-8790/3), and others. The total time period was broken into segments of 1993-1995, 1996-2000, 2001-2005, and 2006-2010. The Surveillance Epidemiology and End Results (SEER) stages (local, regional, distant, and unknown) were accessible from 2005 to 2010.
To describe the characteristics of young-age cancer, we defined it as cancer that developed in patients under the age of forty. We also defined advanced-age cancer as cancer that developed in patients above the age of seventy. We then compared the survivals for patients who developed cancer before the age of forty, at or after the age of forty, before the age of seventy, and at or after the age of seventy. In addition, we examined survival differences between young-age anal cancer, advanced-age anal cancer, and distant metastasis of anal cancer. We also estimated the survival difference between males and females.
We used the 5-year relative survival rate (RSR), adjusted for the mortality expected among the general population of the same age and sex. The RSRs were calculated using the Ederer II method [6].Relative survival analyses were based on an algorithm written in SAS by Dickman [7], with some minor adaptations. Asymmetric observed survival confidence intervals (CIs) were formed from standard errors estimated using Greenwood's method [8] and a log (-log) transformation. RSR confidence limits were derived by dividing the observed survival limits by the corresponding expected survival rate.
RESULTS
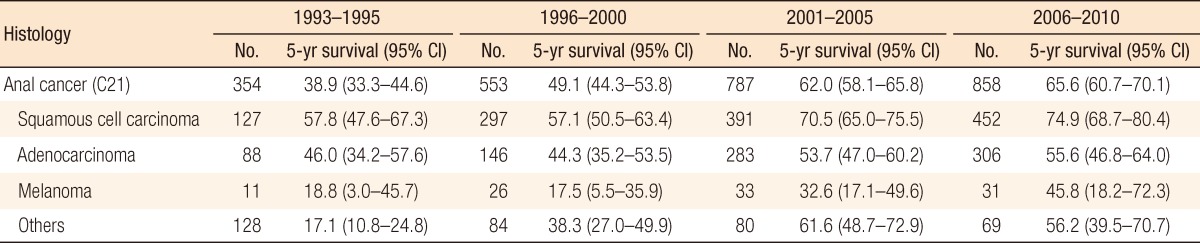
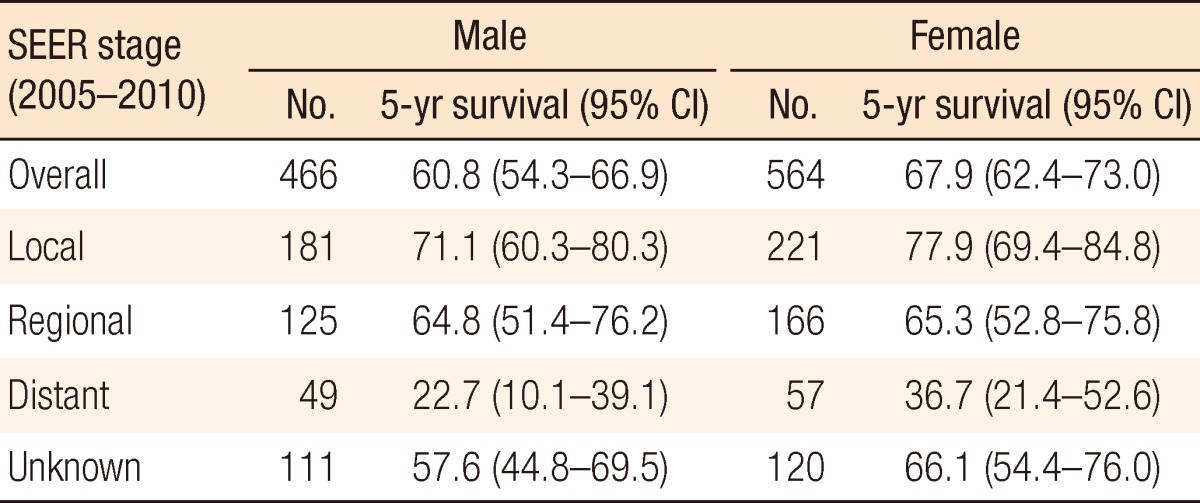
Table 1 shows the 5-year relative survival in cases of anal cancer by time period according to histologic type. The 5-year relative survival increased from 38.9% (95% confidence interval [CI], 33.3-44.6) in 1993-1995 to 65.6% (95% CI, 60.7-70.1) in 2006-2010. According to histologic type, the survivals for all types of anal cancers improved survival with the passage of time. Of the anal-cancer types, survival from melanoma was lower than that from the others. We compared the survival between males and females by using the SEER stage. Our result indicated that females showed better survival than males in all stages, but the difference was not statistically significant (Table 2).
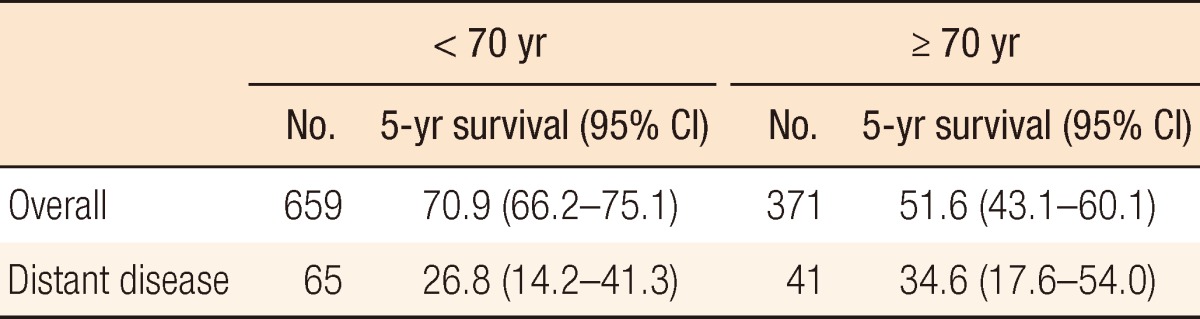
Table 3 shows the 5-year survivals for anal cancer patients whose disease had been diagnosed between 2005 and 2010 in whom the cancer had developed before the age of forty and in whom the cancer had developed at or after the age of forty. Table 4 shows a comparison of the 5-year survival rates for anal cancer patients whose disease had been diagnosed during the same time period in whom the disease had developed before the age of seventy and in whom the disease had developed at or after the age of seventy. Patients in whom the disease had developed at or after the age of seventy showed the worse survival overall, but they showed the best survival in cases where a distant stage of the disease had developed.
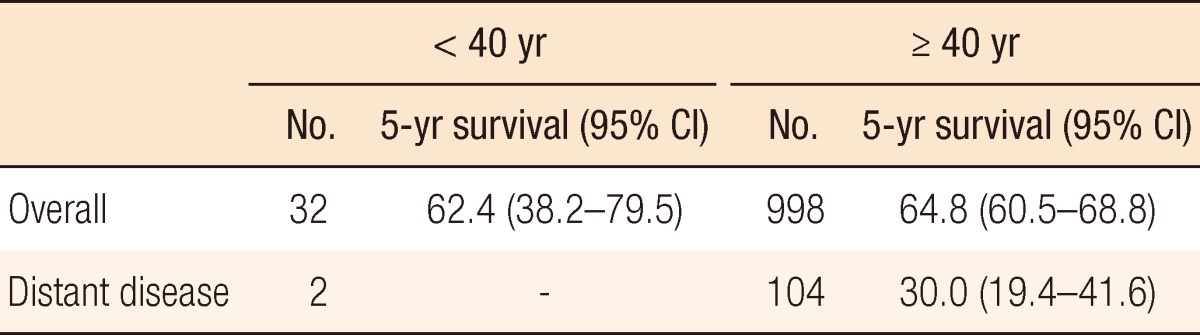
Five-year survival rates in anal cancer according to patients aged < 40 years and ≥ 40 years (KCCR, 2005-2010)
DISCUSSION
Our analysis estimated the survival features of anal cancer in the Korean population based on time period, tumor histology, sex, and specific age at which the cancer developed. Obtaining representative data from the KCCR, we found trends in the survival features of anal cancer in the Korean population.
Radiochemotherapy is the standard treatment for squamous cell carcinomas and has provided a considerable survival rate [8]. The present study also showed more than 70% survival in cases of squamous cell carcinomas from 2001. In addition, a recent study reported the diagnosis of anal cancer in a less advanced stage in United States [9]. This trend may also have an effect on improving survival. However, one limitation of our study was that patients who received surgical treatments were included in the same group as patients who received chemoradiation. Assessing the proportion of patients who underwent surgery would provide a better analysis of survival.
An adenocarcinoma in the anal region is considered as a similar classification of rectal cancer and is the second most common histologic variant. The incidence of this disease entity has remained stable, in contrast to increasing trend of squamous cell carcinomas [9]. The treatment is similar to that for a low rectal adenocarcinoma and includes an abdomino-perineal resection and chemoradiotherapy. This disease entity may be associated with Crohn disease combined with long-standing anal fistulae [10].
An anorectal melanoma is very rare disease in Korea, but is more common in Western countries. Although the survival of patients with an anorectal melanoma is thought to be improved based on our data, it remains a lethal disease with a median survival of less than 2 years. An anorectal melanoma is found in advanced disease and is progressive against multiple treatment modalities or aggressive therapy [11-15]. Nevertheless, radical surgery did have an impact on long-term survival in some studies [16-19].
Gender differences in anal cancer were studied for all SEER stages. Anal cancer in Korea showed a slight predominance in female patients. Homosexual men were at greater risk factor of developing anal cancer. In fact, this mention means these patients are smaller than Western countries. In addition, anal cancer from this etiology is relatively small. As Table 2 shows, females had a better prognosis than men. Although the reason for this gender difference is unknown, female cancer patients in Korea generally demonstrate better survivals for the majority of solid tumors, including anal cancer [20]. Some reports indicate that males seem to have inferior outcomes after chemoradiotherapy for anal squamous cell carcinomas [21, 22].
Survival in young age patients was comparable to the other age groups. In contrast, elderly age had worse survival than other age groups in overall survival. Elderly population is fast growing one in Korea, thus, we consider a particular attention to elderly patients.
Like colorectal cancer, elderly patients were less likely received aggressive therapy for their comorbidities and poor performance state. Some studies suggested standard treatment showed a good efficacy in elderly patients with anal cancer [23-25].
Interestingly, we observed a better survival in elderly patients with distant disease. Although small number of distant disease patients could in part explain this result, we need concern about survival trend.
In summary, although this study is based on the KCCR with limited availability, it provides valuable information about survival characteristics of Korean anal cancer from nationwide database.
ACKNOWLEDGMENTS
This study was supported by the National Cancer Center Grant (NCC-1310220).
Notes
No potential conflict of interest relevant to this article was reported.