Is Microsatellite Instability Really a Good Prognostic Factor of Colorectal Cancer?
Article information
Abstract
Purpose
The aim of this study was to investigate the clinicopathologic features of and the prognosis for colorectal cancers (CRCs) with microsatellite instabilities (MSIs).
Methods
Between 2006 and 2009, genotyping was performed on 245 patients with stage II/III CRCs to establish the MSI status. The clinicopathologic differences and the prognostic value of MSI were analyzed. The median follow-up period was 38 months (range, 7-68 months).
Results
Of the total 245 patients, 20 (8.2%) had MSI-high (H) and 225 (91.8%) had MSI-low (L) or stable (S) CRCs. Adjuvant chemotherapies were performed on 101 stage II (87.8%) and 107 stage III patients (82.3%). Patients with MSI-H CRCs more frequently had a family history of colon cancer (10% vs. 2.7%, P = 0.003), more frequently had a cancer located at the proximal colon (90.0% vs. 19.1%, P < 0.0001), and more often showed a mucinous phenotype or poor differentiation (35.0% vs. 7.1%, P = 0.001). Despite less frequent lymph node metastasis (25% vs. 55.6%, P = 0.01), the number of retrieved lymph nodes was higher (26.3 ± 13.1 vs. 20.7 ± 1.2, P = 0.04) in the MSI-H group. The overall survival and the disease-free survival (DFS) did not differ with respect to MSI status. However, in the stage II subgroup, the DFS for patients with MSI-H CRCs was significantly worse (72.2% vs. 90.7%, P = 0.03). The multivariate analysis performed on this subgroup revealed that MSI-H was an independent poor prognostic factor (adjusted hazard ratio, 4.0; 95% confidence interval, 1.0-15.6, P = 0.046).
Conclusion
MSI-H CRCs had distinct clinicopathologic features, and MSI-H was an independent poor prognostic factor in stage II CRCs. Considering the majority of stage II patients were administrated adjuvant chemotherapy, the efficacy of adjuvant chemotherapy for treating MSI CRCs might be different from that for treating MSI-L/S tumors.
INTRODUCTION
Colorectal cancer (CRC) is one of the leading causes of death in Western countries. However, in Korea, according to 2011 cancer statistics, CRC is the second most common type of cancer in men, and its incidence is rapidly increasing [1]. Traditional pathological staging systems have been useful in predicting the outcome of CRC, but it is now evident that these cancers are actually heterogeneous. The natural history of CRC is strongly linked to genetic alterations that occur during its progression from an adenoma to a carcinoma and from a carcinoma to metastatic disease [2]. Two principal genetic pathways underlie the development of CRC. Most CRC tumors exhibit chromosomal instability associated with the loss of heterozygosity at multiple tumor suppressor loci such as 5q, 17p, and 18q [3]. The microsatellite instability (MSI) refers to the mutational signature found in CRCs that evolve as a result of the inactivation of the DNA mismatch repair (MMR) system and is observed in approximately 15% of all CRCs. Furthermore, approximately 3% of all CRCs arise as a consequence of Lynch syndrome, and nearly all Lynch syndrome CRCs exhibit MSI [4]. Another 12% of CRCs represent the noninherited form of DNA MMR inactivation that is induced by methylation and, hence, silences the MLH1 gene promoter [5]. MSI-high (H) CRCs have a number of distinct features compared to MSI-low (MSI-L) and MSI-stable (MSS) CRCs, with the former being associated, on average, with a younger age at diagnosis and with tumors that more frequently have a mucinous phenotype, a poorly differentiated histology, and a proximal location and that show peritumoral lymphocytic infiltration [6].
Along with these distinguishing clinical characteristics, most previous studies reported that patients with MSI-H CRCs survive longer than do stage-matched patients with cancers exhibiting MSI-L/S [6-11]. Furthermore, the MSI status has been reported to have predictive value for the effectiveness of adjuvant chemotherapy, as for MSI-H patients, adjuvant chemotherapy with 5-fluorouracil (5-FU) has no benefit over surgery alone [12, 13]. Therefore, in this study, we assessed the clinicopathologic features and prognoses of MSI-H CRC patients who underwent curative surgery.
METHODS
In this study, which was approved by the Institutional Review Board of the hospital and in which written informed consent for tissue collection was obtained from all patients, we analyzed a prospectively-collected series of 245 consecutive CRC patients who underwent a curative resection for pathologically-confirmed stages II or stage III CRCs at the Korea Cancer Center Hospital between 2006 and 2009. Patients were excluded if they had hereditary nonpolyposis CRC that met the Amsterdam II criteria, familial adenomatous polyposis, or a previous history of treatment for a malignancy. In order to analyze the MSI status, we amplified DNA extracted from formalin-fixed and paraffin-embedded tissue samples by using a polymerase chain reaction and a previously-recommended panel of markers (bat26, bat25, D5S346, D2S123, and D7S250) for genetic classification [14]. MSI-H was defined as instability in two or more markers, MSI-L as instability in a single marker, and MSS as no evidence of instability in the markers. The pathological staging of cancer was assessed postoperatively according to the seventh edition of the American Joint Committee on Cancer TNM grading system [15]. We described the location of a tumor as being in the proximal colon when it was located proximal to the splenic flexure.
Two 5-FU-based regimens were used for adjuvant chemotherapy. The first was the FL regimen, comprising 5-FU (500 mg/m2) and leucovorin (20 mg/m2) for 5 days every month for 6 cycles. The second was the FOLFOX4 regimen that consisted of 5-FU (400 mg/m2 for loading, 600 mg/m2 for maintenance) and leucovorin (200 mg/m2), each for 2 days every 2 weeks. Oxaliplatin (85 mg/m2) was administered on the first day and repeated for 12 cycles. Patients who were administered more than 50% of the planned schedule of chemotherapy were entered into the adjuvant chemotherapy group. Thirty-seven patients did not receive chemotherapy because of their poor performance status or their refusal to undergo this treatment.
For detection of recurrence during the follow-up period, the carcinoembryonic antigen level and a computed tomography scan were checked every three months for the first year after the operation, and every six months thereafter. The median follow-up duration for all patients was 38 months.
Statistical analysis was performed using SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA). All results in the text and tables are given as mean ± standard deviation. Categorical variables were analyzed using the chi-square test or Fisher exact test. Comparisons of quantitative variables between groups were made using the independent sample t-test. The Kaplan-Meier method was used for survival analysis, and the log rank test was used to determine any difference in survival between the groups. The Cox proportional hazard model with a backward elimination method was used for multivariate analyses. In all cases, a P-value of less than 0.05 was considered statistically significant.
RESULTS
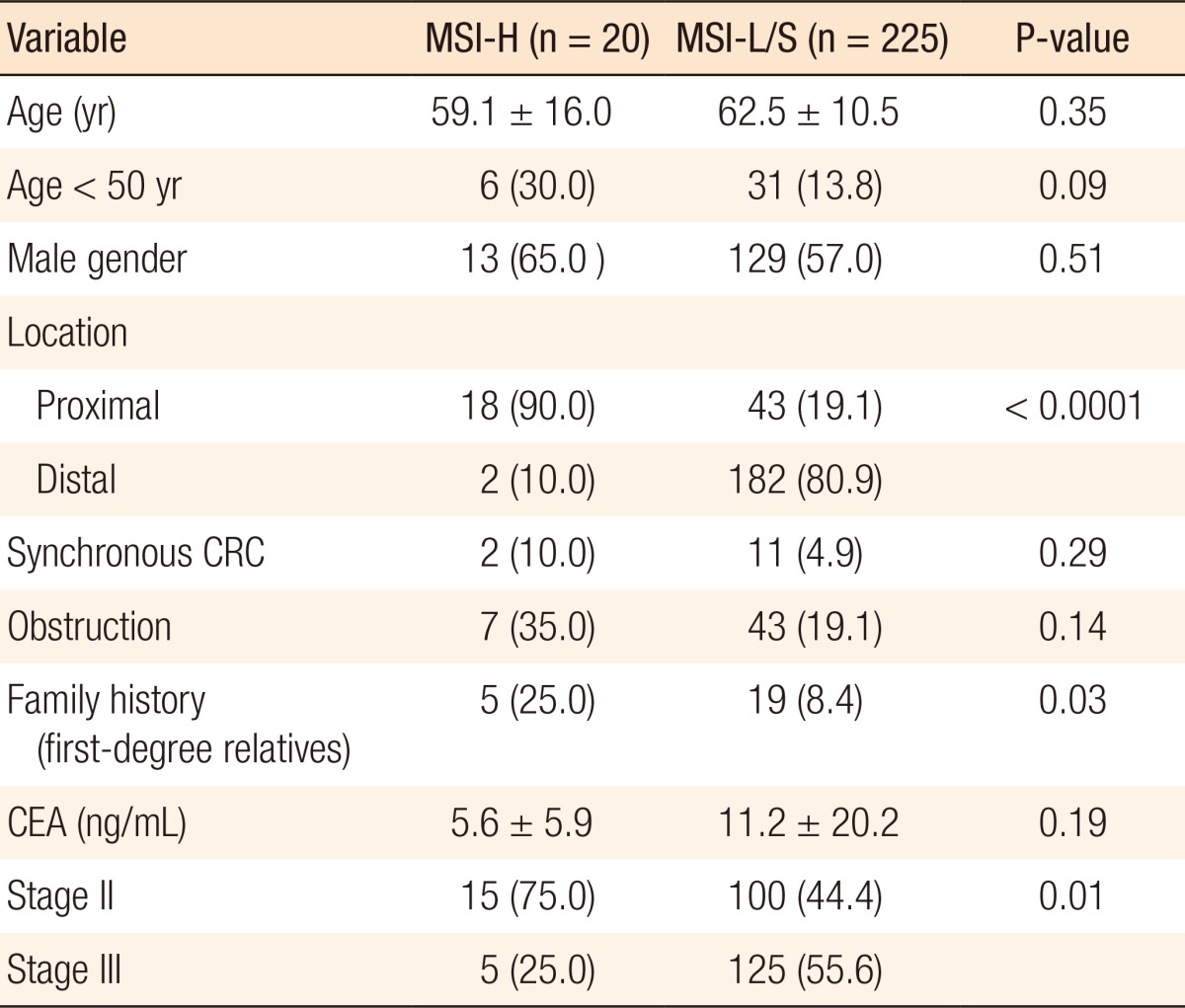
Of the 245 patients included in this study, 20 (8.2%) were found to have MSI-H tumors, and the remaining 225 patients had MSS or MSI-L tumors. Patients with MSI-H tumors were younger on average than were patients with MSI-L/S tumors, with a greater proportion being under 50 years of age, although this difference was not statistically significant. Furthermore, MSI-H primary tumors were located proximal to the splenic flexure significantly more frequently than MSI-L/S tumors (90.0% vs. 19.1%, P < 0.0001), and MSI-H CRC patients more frequently had a family history of colon or colon-related cancer in first-degree relatives (25.0% vs. 8.4%, P = 0.03). A further significant difference was in the lower frequency of lymph node metastasis in patients with MSI-H CRCs (55.6% vs. 25.0%, P = 0.01) (Table 1).
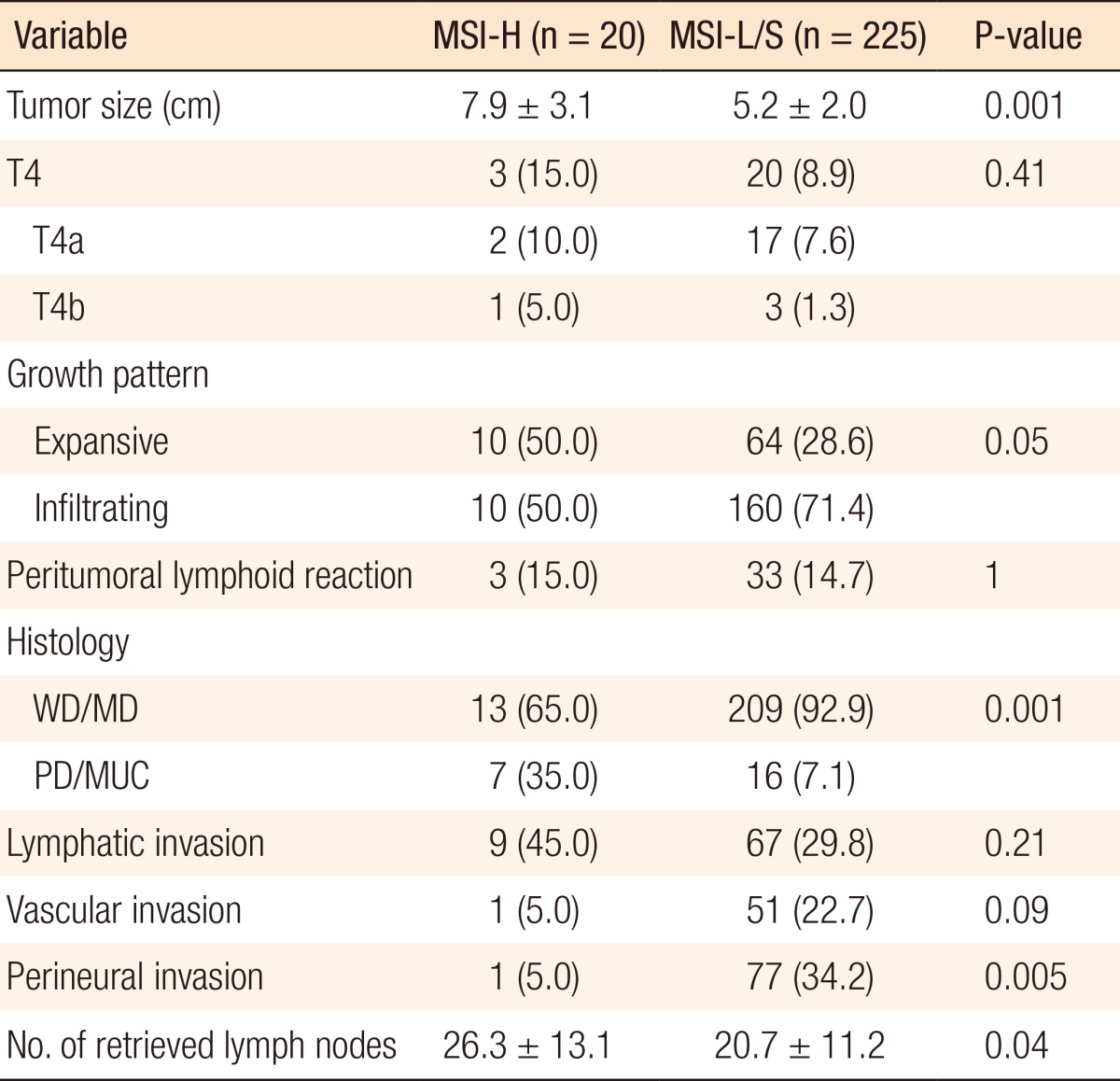
A pathological analysis revealed that the primary tumor size in the MSI-H group was 7.9 ± 3.1 cm, which was significantly larger than that in the MSI-L/S group (5.2 ± 2.0 cm, P = 0.001). MSI-H CRCs showed an expansive growth pattern significantly more frequently (50.0% vs. 28.6%, P = 0.05) than MSI-L/S tumors and more often had a poorly differentiated or a mucin-containing histology (35.0% vs. 7.1%, P = 0.001). The number of retrieved lymph nodes was also significantly higher for MSI-H CRCs than for MSI-L/S CRCs (26.3 ± 13.1 vs. 20.7 ± 11.2, P = 0.04), although the frequency of peritumoral lymphoid reaction was not significantly different between the groups (Table 2).
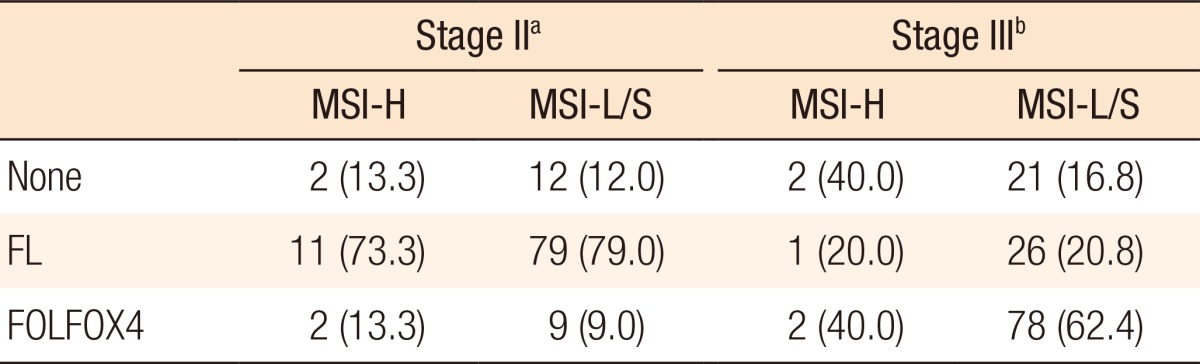
Table 3 summarizes the adjuvant chemotherapy administered to patients with MSI-H CRCs or MSI-L/S CRCs. Thirteen patients (86.7%) received adjuvant chemotherapy for stage II disease, 11 (73.3%) of whom received the FL regimen and 2 (13.3%) received FOLFOX4. Adjuvant chemotherapy was administered to 88 of the patients (87.0%) with MSI-L/S stage II CRC and to a similar proportion of patients with MSI-H stage II CRC. The distributions of the chemotherapy regimens were not significantly different. Of the 5 patients with MSI-H stage III CRC, 1 (20.0%) was treated using the FL regimen, and 2 (40.0%) received FOLFOX4 chemotherapy. In the MSI-L/S group, 104 of the patients (83.2%) received adjuvant chemotherapy, which was FOLFOX4 in 78 of the patients (62.4%). A higher proportion of stage III MSI-L/S CRC patients underwent chemotherapy, although this difference was not statistically significant (P = 0.27).
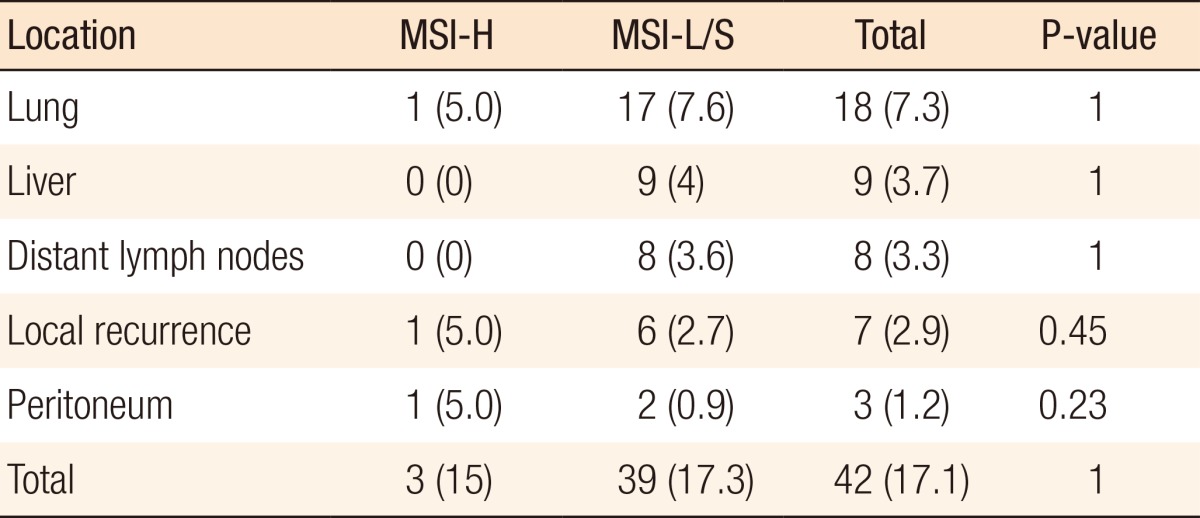
During the follow-up period, 42 patients (17.1%) showed recurrence, most commonly in the lung (n = 17, 7.3%) and liver (n = 9, 3.7%). However, the frequency and the site of recurrence did not differ significantly according to MSI status (Table 4).
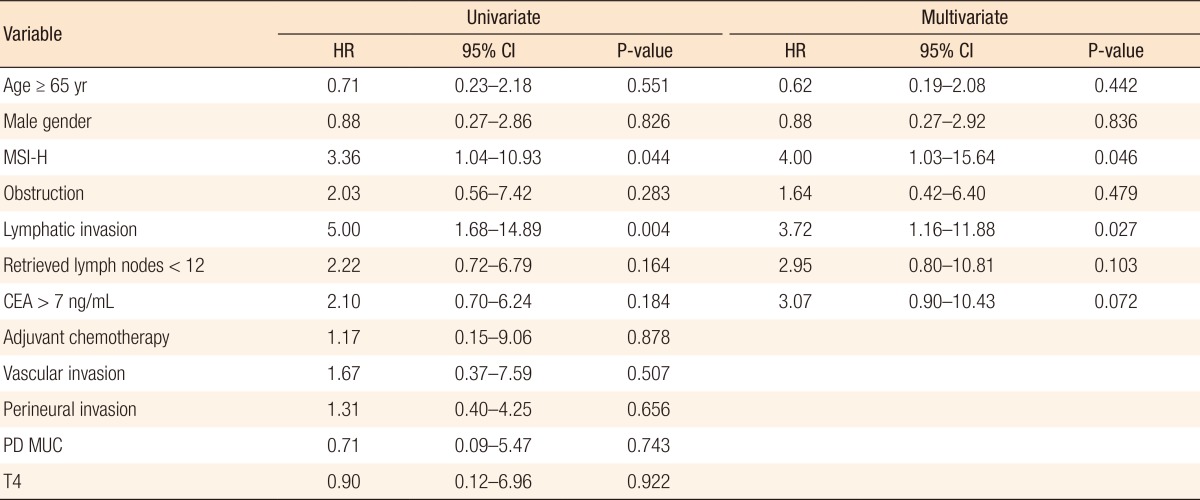
A Kaplan-Meier analysis revealed that the 3-year disease-free survival (DFS) rates of the MSI-H and MSI-L/S groups were 77.5% and 79.6%, respectively (P = 0.78) and that the 3-year overall survival (OS) rates of the 2 groups were similar (87.5% vs. 92.0%; P = 0.95). However, amongst patients with stage II disease, the DFS of the MSI-H group was 72.2%, which was significantly worse than that of the MSI-L/S group (90.7%, P = 0.03) (Fig. 1). The OS of the MSI-H group was also worse, but this difference was not statistically significant (83.3% vs. 95.6%, P = 0.24) (Fig. 2). In the stage III subgroup, the OS and the DFS were longer in the MSI-H group than in the MSI-L/S group, although these differences were not statistically significant (Figs. 3 and 4). A multivariate Cox analysis of the DFS in the stage II subgroup, in which the confounding effect of other risk factors was accounted for, revealed that MSI-H was an independent factor for a poor prognosis (adjusted hazard ratio [aHR], 4.0; 95% confidence interval [CI], 1.0-15.6, P = 0.046), as was lymphatic invasion (aHR, 3.7; 95% CI, 1.16-11.88; P = 0.027) (Table 5).

Kaplan-Meier analysis of disease-free survival in the stage II subgroup. MSI-H, microsatellite instability-high; MSI-L/S, microsatellite instability-low/stable.
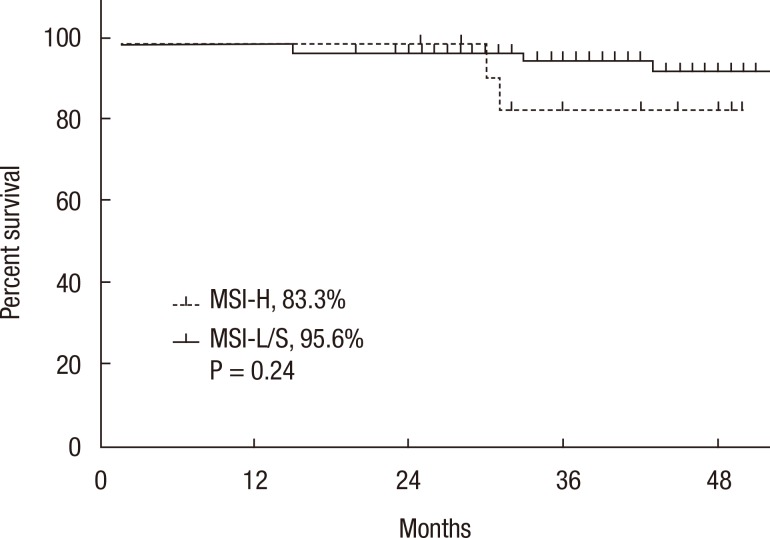
Kaplan-Meier analysis of overall survival in the stage II subgroup. MSI-H, microsatellite instability-high; MSI-L/S, microsatellite instability-low/stable.

Kaplan-Meier analysis of disease-free survival in the stage III subgroup. MSI-H, microsatellite instability-high; MSI-L/S, microsatellite instability-low/stable.
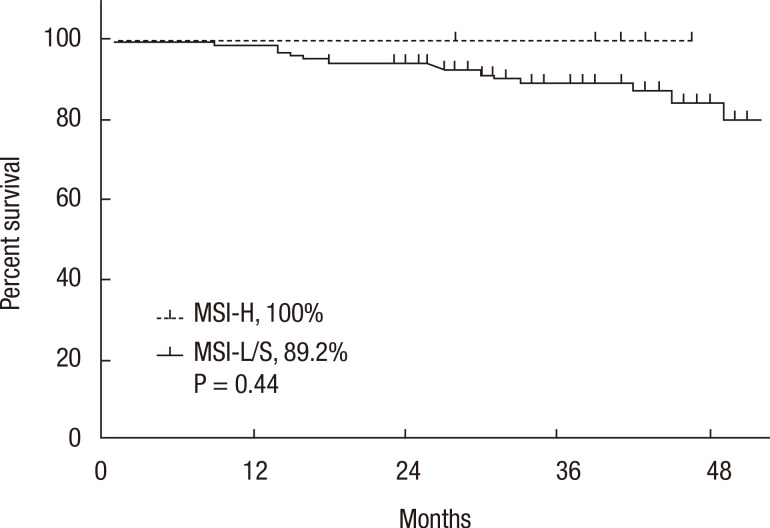
Kaplan-Meier analysis of overall survival in the stage III subgroup. MSI-H, microsatellite instability-high; MSI-L/S, microsatellite instability-low/stable.
DISCUSSION
Following the initial discovery of MSI in patients with Lynch syndrome and in approximately 15% of patients with sporadic CRC tumors, considerable data have been accumulated and have demonstrated an improved outcome for patients with MMR-deficient CRC compared to those with MMR-proficient tumors or tumors with chromosomal instability. Patients with MSI-H tumors have distinct clinical and pathological features, irrespective of their germ line or sporadic origins [16]. These features include proximal colon predominance, frequent poor differentiation, mucinous histology, and an increased number of tumor-infiltrating lymphocytes [6]. In agreement with previous studies, we found that MSI-H CRC cases had different clinical and pathological characteristics compared to MSI-L/S CRC cases. In the former, the tumors were more frequently located in the proximal colon, were larger on average, had an expansive growth pattern more frequently, and were associated with a higher number of retrieved lymph nodes and less frequent lymph node metastasis. MSI-H CRCs were also frequently associated with mucinous histology and poor differentiation.
However, an important finding in our study was that patients with stage II disease and MSI-H tumors had poorer survival outcomes than those with an MSI-S tumor. Most studies, including a recent one on a large consecutive Norwegian series, showed better survival for MSI-H CRCs [17]. However, some reports showed a different result after 5-FU-based adjuvant chemotherapy in patients with MSI-H CRCs [12, 13]. Ribic et al. [12] published a report describing 570 patients with colon cancer enrolled in 5 prior phase III trials of 5-FU-based adjuvant chemotherapy following curative resection for stage II and stage III colon cancer. In their analysis, MSI-H patients not treated with chemotherapy showed significantly improved survival. However, in the group of patients who received adjuvant chemotherapy, MSI-H was not associated with an improved OS (hazard ration [HR], 1.07; 95% CI, 0.62-1.86; P = 0.8), although the benefit of treatment did differ significantly according to the tumor MSI patients with non-MSI-H tumors (HR, 0.72; 95% CI, 0.53-0.99; P = 0.04) [12]. Moreover, Sargent et al. [13] recently confirmed and extended the initial findings of Ribic et al. [12] with a large retrospective study including samples obtained from six randomized, controlled trials. Their study evaluated the benefit of 5-FU-based adjuvant chemotherapy in patients with stage II CRC, excluding regimens combining oxaliplatin, irinotecan, and oral fluoropyrimidines. Stage III patients were not included in that analysis as adjuvant chemotherapy was already the standard of care. The authors concluded that 5 FU did not benefit the patients and that, indeed, it might even be harmful for stage II CRC patients with MSI tumors. In the present study, we performed adjuvant chemotherapy to treat stage II CRC (87.8%) as often as we did to treat stage III CRC (82.3%). Considering that the majority of stage II patients were administrated the adjuvant FL regimen, the poor outcome for stage II MSI-H CRCs might be attributable to a relationship between the efficacy of adjuvant 5-FU-based chemotherapy and MSI status. Therefore, we assume that the nonbeneficial effect of 5-FU for MSI-H stage II patients and the improved survival of MSI-L/S patients who were administered the same adjuvant chemotherapy might make a significant contribution to a worse DFS. A multivariate analysis of the stage II subgroup revealed that MSI-H, after adjusting for other high risk factors, was an independent factor for a poor prognosis. A recent study about the use of molecular biomarkers for making adjuvant therapy decisions in stage II CRCs showed results in accordance with our findings. According to their report, while the MMR status had no influence on cancer progression in the surgery-alone group, defective MMR, along with CpG island methylation phenotype status, was the most important predictor of poor survival in the Cox regression analysis of the 5-FU treatment group [18].
The issue about adjuvant chemotherapy to treat stage II CRC has been controversial. Nevertheless, we have recommended adjuvant FL chemotherapy to treat stage II colon cancer for the low-risk group and both FOLFOX and FL regimens for high-risk patients for following reasons: In the MOSAIC trial, which evaluated the benefit of the adjuvant FOLFOX over the FL regimen, recommended FOLFOX for high-risk stage II patients [19]. The characteristics of high-risk patients are T4 tumors; tumors with a low histological grade (for example, poorly differentiated and undifferentiated carcinomas); lymphatic, vascular or neural invasion; obstruction or perforation; and less than 10 harvested lymph nodes. Also, the QUASAR study did show a significant benefit of using adjuvant chemotherapy with 5-FU for stage II colon cancer [20], and our previous report on adjuvant chemotherapy to treat stage II colon cancer showed that stage II patients who received adjuvant chemotherapy with the FL regimen had significantly improved survival over the nonchemotherapy patients in both the low and the high risk of recurrence groups [21].
Several biological mechanisms have been proposed to explain why 5-FU-based adjuvant chemotherapy fails to benefit patients with a defective MMR system. These include an antitumor immune response involving the lymphocytic infiltration characteristic of MSI-H tumors [22], which may be diminished by the immunosuppressive effects of chemotherapy. In vitro studies have also predicted a differential efficacy of 5-FU with respect to MMR status. Those studies found a recovery of chemoresistance to 5-FU for hypermethylated defective MMR CRC cell lines that had been treated with demethylating agents [23, 24]. A second possibility relates to the role of MMR systems in the removal of 5-FU from DNA, whereby the absence of these systems may reduce DNA repair and, thus, attenuate the effect of 5-FU [25]. It has been suggested that adding oxaliplatin to this regimen may overcome the negative effect of 5-FU on the DFS of patients with MSI-H CRCs [26]. In our study, despite more MSI-L/S patients with stage III disease receiving FOLFOX4 chemotherapy, there was no significant difference in survival. Although our findings need to be interpreted with caution because of the small number of patients, survival was better in the MSI-H patients. Whether the lack of benefit from adjuvant 5-FU in patients with MMR-defective tumors translates into a lack of benefit from FOLFOX has not been conclusively established. However, several studies have reported that MMR deficiency is not prognostic of the outcome in CRC patients who have received FOLFOX and even showed an improved outcome when using FOLFOX versus 5-FU alone [26, 27].
The major limitation of our study is the small number of enrolled MSI-H CRC cases, which made subgroup analysis difficult. Only 16 MSI-H patients received adjuvant chemotherapy; thus, we did not have enough cases to subgroup the patients according to stage, treatment with adjuvant chemotherapy, kind of chemo-regimen, and other clinico-pathological risk factors for recurrence. Therefore, the predictive value of MSI-H tumors for adjuvant chemotherapy could not be evaluated with satisfactory statistical power in either the univariate or the multivariate analysis. Indeed, the frequency of MSI-H CRC in Korea has been lower than the frequencies previously reported by Western countries [28]. According to a review of this topic from Western countries, evaluating the effect of adjuvant chemotherapy on MSI-H cancers is difficult because of the low number of sporadic MSI-H CRC, resulting in a limited statistical significance for MSI-H patients. The conflicting evidence on the benefit of 5-FU chemotherapy for treating MSI-H CRC patients relies mainly on a retrospective case series in which selection bias was a potential confounder [29]. Therefore, in Korea, practically, revealing the predictive role of MSI-H CRCs in adjuvant chemotherapy will be difficult based on only our single-center experience.
In summary, we identified a number of clinical and pathological features that differed significantly between CRC patients with and without MSI-H tumors. Also, MSI-H CRC showed worse survival results and an independent poor prognostic factor in stage II patients. We assume that these results might be due to 5-FU therapy having a different effect on MSI-H CRCs. However, this single-institutional retrospective study with a limited number of cases cannot be used to conclude that 5-FU-based adjuvant chemotherapy is actually of no benefit for patients with MSI-H CRCs. We believe that a large-scale well-designed multicenter study would be able to identify those features of MSI-H CRCs that can act as prognostic and predictive markers.
Notes
No potential conflict of interest relevant to this article was reported.