Recurrence after endoscopic resection of small rectal neuroendocrine tumors: a retrospective cohort study
Article information
Abstract
Purpose
According to the European Neuroendocrine Tumor Society consensus guidelines, rectal neuroendocrine tumors (NETs) up to 10 mm in size and without poor prognostic factors could be safely removed with endoscopic resection, suggesting omitting surveillance colonoscopy after complete resection. However, the benefit of surveillance colonoscopy is still unknown. In this study, we aimed to report the outcomes after endoscopic resection of small rectal NETs using our surveillance protocol.
Methods
This retrospective cohort study included patients who underwent endoscopic resection for rectal NETs sized up to 10 mm from January 2013 to December 2019 at our center. We excluded patients without surveillance colonoscopy and those lost to follow-up. We strictly performed surveillance colonoscopy 1 year after endoscopic resection, and every 2 to 3 years thereafter. The primary outcomes were tumor recurrence and occurrence of metachronous tumors during follow-up.
Results
Of the 54 patients who underwent endoscopic resection for rectal NETs during the study period, 46 were enrolled in this study. The complete resection rates by endoscopic mucosal resection, precutting endoscopic mucosal resection, and endoscopic submucosal dissection were 92.3% (12 of 13), 100% (21 of 21), and 100% (12 of 12), respectively. There was no local or distant recurrence during the median follow-up of 39 months. However, we found that 8.7% (4 of 46) of patients developed metachronous NETs. All metachronous lesions were treated with precutting endoscopic mucosal resection.
Conclusion
Surveillance colonoscopy is reasonable after endoscopic resection of small rectal NETs for timely detection and treatment of metachronous lesions. However, larger collaborative studies are needed to influence the guidelines.
INTRODUCTION
Neuroendocrine tumors (NETs) are rare neoplasms that originate from the Kulchitsky cells (i.e., enterochromaffin cells) located in the crypts of Lieberkuhn. Rectal NETs are known to have the best prognosis of all NETs, with an 88.2% 5-year survival rate [1]. They represent 16% of all NETs and 27% of gastrointestinal NETs [2]. The Surveillance, Epidemiology, and End Results registry database of the National Cancer Institute shows that the age-adjusted incidence of rectal NETs has increased about tenfold over the last 35 years [3]. The rapid increase in incidence might be associated with the increased use of colorectal cancer screening [4]. Screening colonoscopy leads to the detection of rectal NETs of smaller size and earlier stage; 93% to 100% of rectal NETs detected with screening colonoscopy are less than 10 mm in size [1, 5].
According to the European Neuroendocrine Tumor Society (ENETS) consensus guidelines for the management of colorectal NETs, rectal NETs up to 10 mm in size and without poor prognostic factors (G3, lymphovascular invasion [LVI], and muscularis propria invasion) could be safely removed with endoscopic resection because of the low risk of metastasis (2%) [6, 7]. Endoscopic mucosal resection (EMR), precutting EMR (P-EMR), and endoscopic submucosal dissection (ESD) were reported as effective endoscopic treatment [8-13]. Endoscopists usually select the resection technique based on their preference.
Tumor recurrence after endoscopic resection for rectal NETs of up to 10 mm is uncommon (0%–4.2%) [14-17]. Moreover, the 5-year overall survival was reported to be 95% to 100% [5, 18, 19]. The ENETS guidelines suggest omitting surveillance colonoscopy after complete resection of rectal NETs less than 10 mm in size [7]. Similarly, the National Comprehensive Cancer Network (NCCN) guidelines for NETs of the gastrointestinal tract state that surveillance colonoscopy is not required after resection of rectal NETs smaller than 10 mm [20]. In previous studies with the long-term outcome of rectal NET resection, not all patients underwent regular surveillance colonoscopy [10, 21-24].
In this study, we report the outcome of endoscopic resection for rectal NETs up to 10 mm in size using our surveillance protocol, with focus on the tumor recurrence and occurrence of metachronous tumors. According to our surveillance protocol, surveillance colonoscopy is performed 1 year after endoscopic resection and every 2 to 3 years thereafter. Additionally, patients with high-risk features (NETs grade of ≥ 2 and size of ≥ 10 mm) undergo annual surveillance abdominal computed tomography (CT).
METHODS
Study design and population
Patients who underwent endoscopic resection for rectal NETs at the Surgical Endoscopy Colorectal Division of King Chulalongkorn Memorial Hospital in Bangkok, Thailand from January 1, 2013 to December 31, 2019 were recruited prospectively within the electronic database. We retrospectively analyzed the data from this cohort, including patients’ characteristics, endoscopic characteristics, pathologic reports, and follow-up information. Patients who did not undergo surveillance colonoscopy and those who were lost to follow-up were excluded from the analysis.
Due to the retrospective design of the study, consent was waived by the ethics committee for the entire study. The study protocol was approved by the Institutional Review Board of Chulalongkorn University (No. 196-63).
Endoscopic resection
Endoscopic resection for rectal NETs sized up to 10 mm was usually performed during the initial colonoscopy by the attending staff of the colorectal unit. Abdominal CT was performed after resection because the risk for metastasis for these tumors is very low. Because endoscopic ultrasound is not available in our unit, we selected the endoscopic resection technique according to the tumor size and endoscopic morphology (Fig. 1).
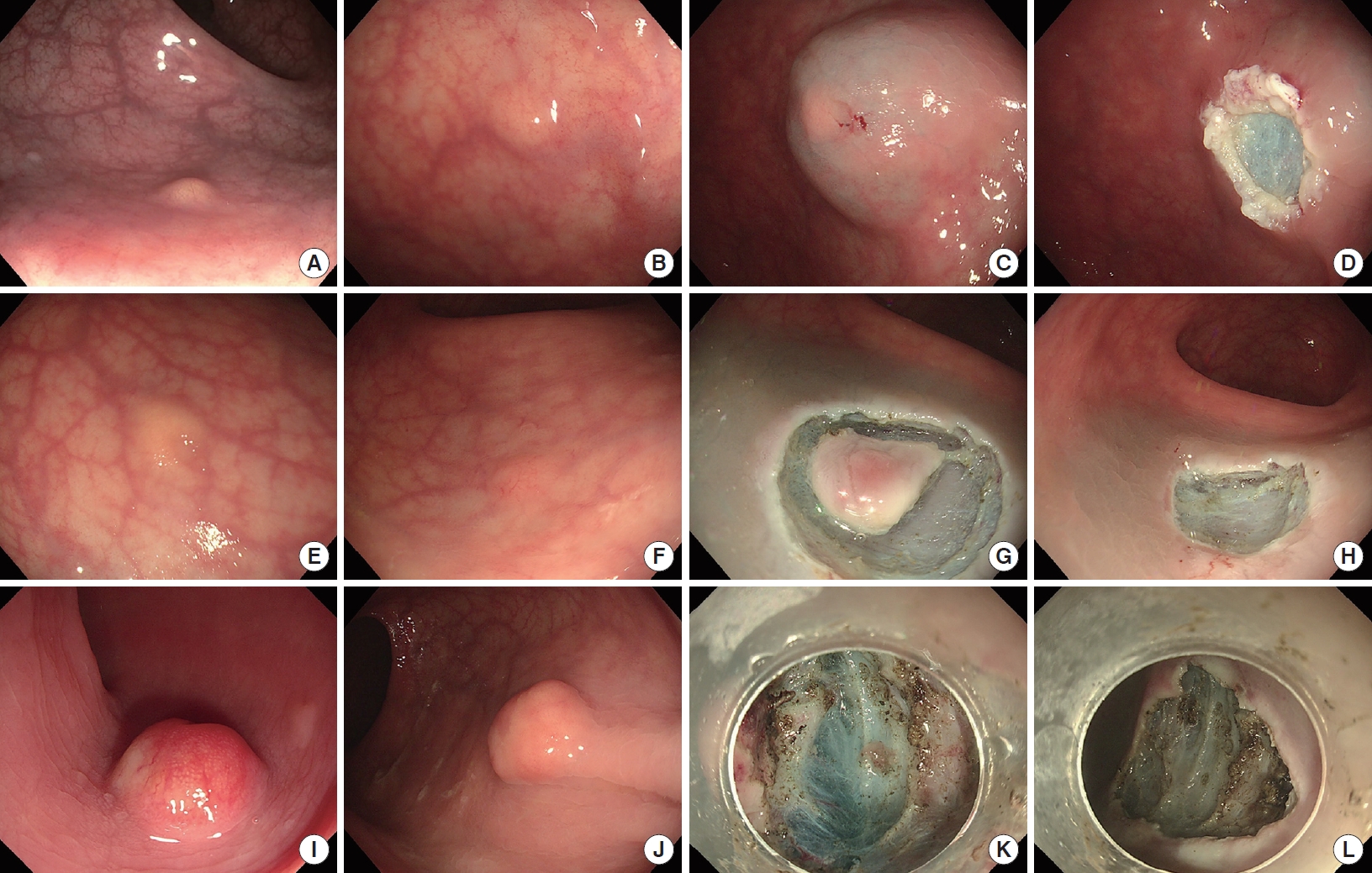
Criteria for selection of an endoscopic resection technique in our institution. (A–D) Endoscopic mucosal resection (EMR), (E–H) precutting EMR (P-EMR), and (I–L) endoscopic submucosal dissection (ESD). (A, B) Rectal neuroendocrine tumors (NETs) of ≤ 5 mm in the superficial submucosa. (C) Lifting. (D) Wound after snaring. (E, F) Rectal NETs of ≤ 5 mm in the deep submucosa. (G) Circumferential incision. (H) Wound after P-EMR. (I, J) Rectal NETs 6 to 10 mm. (K) Submucosal dissection. (L) Wound after ESD.
For EMR, after submucosal saline injection (NeedleMaster injection needle, Olympus Corp., Tokyo, Japan), a 10-mm snare (SnareMaster, Olympus Corp.) was used for resection. Finally, we performed endoscopic clipping (EZ clip, Olympus Corp.) in all cases.
For P-EMR, we performed a circumferential incision to the submucosa around the lesion using an endoscopic knife (Dual Knife, Olympus Corp.) before snare resection.
ESD was primarily performed using the Dual Knife. We used glycerol for submucosal lifting. A transparent distal cap was used from the start of ESD to provide countertraction for dissection.
Pathological evaluation
Complete resection was defined as an en bloc resection without lateral or vertical margin involvement on pathological assessment. All resected specimens were examined with H&E and immunohistochemical staining with synaptophysin and chromogranin. The mitotic count and Ki-67 index were measured. LVI was evaluated. Grading of rectal NETs was performed according to the 2010 World Health Organization (WHO) classification [25].
Outcomes and definitions
The primary outcome was tumor recurrence, including local and distant recurrence and the occurrence of metachronous NETs. Secondary outcomes included complete resection rate, procedural time, and complications.
Local recurrence was defined as development of NETs adjacent to the previous scar after endoscopic resection. Distant recurrence was defined as development of NETs beyond the rectal wall. Synchronous rectal NETs were defined as more than 1 rectal NETs in a patient detected on colonoscopy up to 6 months after diagnosis. Metachronous rectal NETs were defined as NETs detected at a site distant from the primary lesion more than 6 months after initial diagnosis. For the evaluation of metachronous lesions, a cutoff period of 6 months after initial diagnosis, was used to exclude initial tumors that had missed or local recurrence from incomplete excision.
Follow-up protocol
We used our own surveillance protocol because there is no well-established surveillance strategy. All patients underwent surveillance colonoscopy 1 year after endoscopic resection and every 2 to 3 years thereafter. Additionally, patients with high-risk features (NETs grade of ≥ 2 and size of ≥ 10 mm) underwent annual surveillance abdominal CT.
Statistical analysis
The data were analyzed using Stata ver. 15.1 (Stata Corp., College Station, TX, USA). Continuous variables were assessed by plotting histograms to assess whether data were normally distributed. They were compared using one-way analysis of variance, followed by Bonferroni post hoc comparisons, Kruskal-Wallis rank test, and Wilcoxon rank-sum test as appropriate. Categorical variables were compared using two-tailed chi-square tests or Fisher exact test. A P-value of < 0.05 was considered to be statistically significant.
RESULTS
During the study period, 54 patients underwent endoscopic resection for rectal NETs. Three patients were lost to follow-up, and 5 patients did not undergo surveillance colonoscopy. Therefore, 46 patients were included in this study (Fig. 2).

Flowchart of enrolled patients and recurrence after endoscopic resection for rectal neuroendocrine tumors (NETs). EMR, endoscopic mucosal resection; P-EMR, precutting EMR; ESD, endoscopic submucosal dissection.
Table 1 shows the patients’ characteristics. EMR, P-EMR, and ESD were performed in 13, 21, and 12 patients, respectively. The overall mean age of the patients was 59 years (range, 38–78 years). There was a significant difference among the groups regarding the tumor size (P=0.003); tumors in the ESD group were significantly larger than those in the EMR and P-EMR groups (P=0.007 and P=0.009, respectively). The follow-up period in the EMR group was longer than that in the P-EMR and ESD groups (P<0.001 and P=0.033, respectively). Only 1 patient (2.2%, 1 of 46) had multiple synchronous rectal NETs on initial colonoscopy. After pathologic evaluation, only 1 patient in the EMR group had a grade 2 NET based on the WHO classification; the remaining patients had grade 1 NETs. None of the patients had LVI or regional lymph node or distant metastasis on abdominal CT.
The clinical outcomes in each endoscopic resection modality are summarized in Table 2. The complete resection rate was 100% in the P-EMR and ESD groups, and 92.3% (12 of 13) in the EMR group. The deep margin of one patient in the EMR group could not be assessed due to the cauterized effect. After discussion with the patient, we decided to do a close follow-up; there was no recurrence during a follow-up of 86 months. The procedural time was longer in the ESD group than that in the EMR and P-EMR groups (P<0.001 and P=0.013, respectively), and the procedural time of the P-EMR group was longer than that of the EMR group (P<0.001). No complication occurred in any of the groups.
Table 3 shows the tumor recurrence and occurrence of metachronous tumor in each endoscopic resection modality. After endoscopic resection, there was no local or distant recurrence during the median follow-up of 39 months (range, 12–86 months). However, we found that 8.7% (4 of 46) of patients developed metachronous rectal NETs. The median time to metachronous NETs was 41.5 months (range, 19–47 months). The mean diameter of the metachronous lesions was 4 mm (range, 3–7 mm). All metachronous NETs were resected with P-EMR and classified as grade 1 NETs. The characteristics of all patients with metachronous NETs are described in more detail in Table 4.
DISCUSSION
In this study, we analyzed the outcomes of endoscopic resection for rectal NETs up to 10 mm in size using our surveillance protocol based on surveillance colonoscopy performed 1 year after endoscopic resection and every 2 to 3 years thereafter. During the median follow-up period of 39 months, there was no local or distant tumor recurrence; however, 8.7% of the patients developed metachronous rectal NETs.
Previous studies showed that the recurrence rate after endoscopic resection for rectal NETs was low (0%–4.2%) [14-17, 21, 24, 26-28]. There was a diminutively increased chance of local recurrence with a positive resection margin. In a multicenter study, Moon et al. [29] found that local recurrence occurred in 0.74% of 407 patients who underwent endoscopic resection. They had 18.7% positive and 17.7% indeterminate margins. While Chung et al. [30] reported that no local recurrence occurred with 3.9% positive resection margins. Similarly, our results showed no local recurrence with 2.2% positive resection margins.
Few studies have reported metachronous NETs on surveillance colonoscopy. Moon et al. [29] found that 0.74% of patients developed metachronous NETs during a median follow-up of 45 months. Kwak et al. [24] detected only 1 case (1%) of metachronous NETs during 84 months of follow-up. Recently, Chung et al. [30] reported that 9 of 329 patients (2.7%) with rectal NETs of up to 1 cm developed metachronous NETs after endoscopic resection. Our study had a much higher rate (8.7%) of metachronous rectal NETs compared with those studies. The pathogenesis of metachronous NETs is still unknown. Chung et al. [30] found an association between development of metachronous lesions and the presence of synchronous lesions at initial diagnosis (hazard ratio, 1.75; P<0.001). However, in our study, only 1 patient had synchronous lesions, and this patient did not develop a metachronous lesion during the follow-up period.
The NCCN guidelines for NETs of the gastrointestinal tract state that surveillance colonoscopy is not required after resection of rectal NETs less than 10 mm in size [20]. Similarly, the ENETS consensus guidelines for colorectal neuroendocrine neoplasms do not recommend surveillance colonoscopy for rectal NETs sized less than 10 mm treated with complete resection [7]. Although these guidelines do not recommend it, many endoscopists still perform surveillance colonoscopy. The results of our study indicate the potential benefit of surveillance colonoscopy. We detected 8.7% of metachronous NETs during the 39-month median follow-up. The mean diameter of metachronous NETs was 4 mm, and we resected them with P-EMR. If surveillance colonoscopy was not performed, they could progress to larger NETs requiring radical surgery. However, the cost-effectiveness and the interval of surveillance colonoscopy are still unknown. Additional larger studies are needed to address the role of surveillance colonoscopy after endoscopic resection of rectal NETs.
Rectal NETs less than 10 mm in size with no muscularis propria invasion or lymph node metastasis are the indication for endoscopic resection [7, 20]. However, there is no specific recommendation on the optimal endoscopic resection technique for rectal NETs. Endoscopists usually select the resection technique based on their preference. Among the available techniques, EMR is simple and quick, but it has the lowest pathological complete resection rate (28.6%–82.1%) [8-11]. P-EMR is a modification of EMR and has a better pathological complete resection rate (81.2%–96.7%) [9, 12, 13, 26, 27]. Although ESD has a pathological complete resection rate of 77.8% to 100%, it is technically difficult and has a higher rate of complications [8, 10, 12, 21, 26, 27]. In our study, we selected the endoscopic treatment modality based on the tumor size and endoscopic morphology due to the unavailability of endoscopic ultrasound. If the tumors were less than 5 mm and located in the deep submucosa, we selected P-EMR. For tumors located in the superficial submucosa, we performed EMR. ESD was reserved for NETs sized 6 mm or more. The pathological complete resection rates of EMR, P-EMR, and ESD in this study were 92.3%, 100%, and 100%, respectively, and there were no complications. Therefore, we believe that the tumor size and endoscopic morphology could be considered to select the endoscopic treatment modality when endoscopic ultrasound is not available.
This study has several limitations. First, 14.8% of patients (8 of 54) were excluded from the study because they had not undergone surveillance colonoscopy or were lost to follow-up. The rate of metachronous NETs might have been lower if a higher rate of patients adhered to the surveillance protocol. Second, the number of patients was small. We had only one patient with synchronous rectal NETs; thus, an association analysis between synchronous and metachronous rectal NETs was impossible. Third, 4 endoscopists performed endoscopic resection in this study. We cannot avoid the variation of competency in endoscopic evaluation and treatment among our staff. However, most procedures (37 of 46, 80.4%) were performed by S.K., who has performed > 10,000 colonoscopies and > 400 ESD procedures. Fourth, the metachronous NETs could be the overlooked lesions on the initial colonoscopies because they are small with 4-mm mean diameter, which is difficult to prove. However, the initial colonoscopies of all the patients with metachronous NETs were performed by S.K., who is the most experienced endoscopist in this center. Finally, the median follow-up time was 39 months in this study. Indeed, studies with a longer follow-up duration are needed to address the incidence of metachronous rectal NETs.
In conclusion, endoscopic resection is effective for rectal NETs up to 10 mm in size. Despite the absence of local or distant recurrence in this study, the incidence of metachronous rectal NETs was higher than that reported in previous studies. Therefore, surveillance colonoscopy is reasonable after endoscopic resection for timely detection and treatment of metachronous rectal NETs. However, larger collaborative studies are required to influence the guidelines.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.