Robotic Intersphincteric Resection for Low Rectal Cancer: Technical Controversies and a Systematic Review on the Perioperative, Oncological, and Functional Outcomes
Article information
Abstract
Intersphincteric resection (ISR) is the ultimate anus-sparing technique for low rectal cancer and is considered an oncologically safe alternative to abdominoperineal resection. The application of the robotic approach to ISR (RISR) has been described by few specialized surgical teams with several differences regarding approach and technique. This review aims to discuss the technical aspects of RISR by evaluating point by point each surgical controversy. Moreover, a systematic review was performed to report the perioperative, oncological, and functional outcomes of RISR. Postoperative morbidities after RISR are acceptable. RISR allows adequate surgical margins and adequate oncological outcomes. RISR may result in severe bowel and genitourinary dysfunction affecting the quality of life in a portion of patients.
INTRODUCTION
Intersphincteric resection (ISR) is the ultimate anus-preserving technique for the surgical treatment of low rectal cancer (within 5 cm from the anal verge, AV) [1]. ISR is a safe oncological alternative to abdominoperineal resection (APR) with the upturn of anus-preservation [2-5]. ISR requires a thorough knowledge of the deep pelvic anatomy [6, 7], and careful patient selection after a critical evaluation of the indication criteria [8-12]. Minimally invasive approaches for ISR provide adequate perioperative, oncological, and functional results compared to open ISR (OISR) [13-16]. Shin et al. [17] performed a retrospective comparative study between OISR and minimally invasive ISR (laparoscopic ISR, LISR; robotic ISR, RISR) on a large series (n=313) of low rectal cancer demonstrating no difference in 5-year overall (OS) and disease-free survival (DFS).
Few authors, especially from East Asian countries, have published studies on RISR. The robotic platform, through the da Vinci Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA, USA), was intentionally developed to overcome the technical limitations of the laparoscopic approach by providing better ergonomics, eliminating physiologic tremors, adding an extra working arm, improving dexterity through articulated instruments with 7 degrees of freedom (EndoWrist Instruments, Intuitive Surgical, Inc.), and introducing a surgeon’s controlled magnified 3-dimensional stereoscopic stable camera [18]. The robotic platform was demonstrated to compensate for surgical difficulty when performing surgery on rectal cancer [19, 20].
The robotic platform provides an optimal view, an effective hemostasis during levator-sphincter dissection, and an efficient traction allowing meticulous pelvic dissections during ISR [6]. Moreover, Kim et al. [15] reported that the robotic approach facilitates an efficient anus-preserving resection in patients with low rectal cancers compared to the open approach. The robotic approach was reported to be the most significant parameter for ISR achievement (odds ratio [OR], 3.467; 95% confidence interval [CI], 2.095–5.738; P<0.001). The robotic approach can provide higher rates of subtotal/total ISR (advanced ISR) compared to the open approach (47.8% vs. 20.2%, P<0.001) with a significantly lower anastomotic level (P<0.001).
Despite the spreading of the robotic approach for rectal cancer treatment, RISR for low rectal cancer is still under evaluation.
This study aims to report the technical aspects of RISR and to perform a systematic review of perioperative, oncologic, and functional outcomes of RISR for low rectal cancer. The final aim is to provide the current state-of-the-art and to draw considerations on low rectal cancer treatment through RISR.
METHODS
The systematic review was conducted according to the 2020 guidelines of the PRISMA Statement (Preferred Reporting Items for Systematic reviews and Meta-analyses) [21]. All the PRISMA steps were performed by 2 authors independently (GNP and SHK). Any discrepancy and the final decision on eligibility were solved by consensus.
Search strategy and eligibility criteria
Identification
A systematic literature search was performed in PubMed, Embase, and Cochrane Library databases. The following combination of terms was searched: (“robotic”) AND (“intersphincteric resection” OR “sphincter preserving surgery” OR “anal preserving surgery” OR “anus preserving surgery” OR “sphincter saving surgery”). Restriction was applied to include only human studies and published up to August 6, 2021. The titles of the retrieved studies were scrutinized, and duplicates were removed. Reference lists from the retrieved manuscripts were reviewed to identify additional relevant articles.
Screening
The studies were screened at the title and abstract level excluding those not pertinent to the study question. Nonretrievable studies were excluded.
Eligibility
A full-text assessment was performed. Studies reporting patients undergoing RISR for low rectal cancers were included. Studies were excluded if: (1) the study design was editorials, commentaries, reviews, technical notes, letters to the editor, or video articles; (2) the type of publication was a conference abstract; and (3) full text was written in other than the English language. In the case of more than 1 study published by the same authors with overlapping data or periods, the latest study with the most adequate design and extended patient series was considered for the review.
Data extraction
The following study and patients’ data were collected: first author, publication year, country where the study was performed, study period, study design, sample size, age, sex, body mass index, distance from the AV, neoadjuvant chemoradiation (nCRT), approach to ISR, type of ISR, estimated blood loss, perioperative transfusions, operation time, conversion rate, protective stoma, cancer stage, distal resection margin (DRM), circumferential resection margin (CRM), positivity of the DRM and CRM, retrieved lymph nodes (LNs), postoperative hospital stay, time to first flatus and to normal diet.
The following study outcomes were collected: 30-day morbidities, complication grading according to the Clavien-Dindo (CD) classification [22], type of morbidity, permanent stoma, mortality (with causes), functional results, duration of follow-up, local and systemic recurrence rate, and OS and DFS rates. Data extraction was performed independently by 2 authors (GNP and SHK). Any discrepancy and the final decision on data were solved by consensus. Missing data was reported as NR (not reported). An Excel database (ver. 15.21.1, Microsoft, Redmond, WA, USA) was used for data recording.
Methodological quality appraisal
The critical appraisal of study quality (biases risk assessment) was performed by 2 authors independently (GNP and SHK) according to the Joanna Briggs Institute critical appraisal tools for case series, case reports, and case-control studies [23]. No predetermined criteria for exclusion were defined. Any discrepancy and the final decision were solved by consensus.
Statistics
Categorical data were expressed as absolute values and/or pooled percentages. Continuous data were expressed as absolute mean/median values with ranges. Calculations were computed with IBM SPSS Statistics for Macintosh, ver. 26 (IBM Corp., Armonk, NY, USA).
RESULTS
Study characteristics
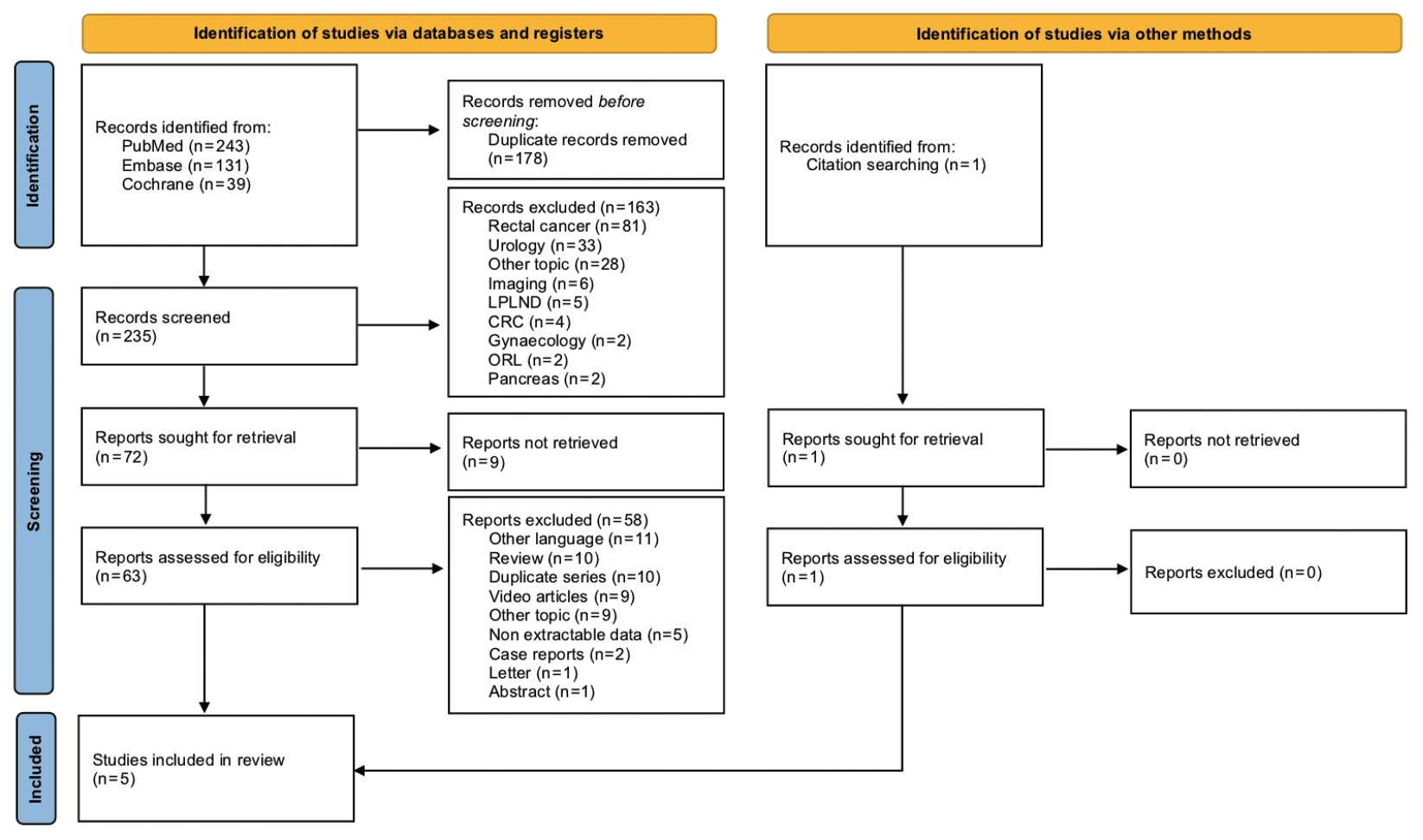
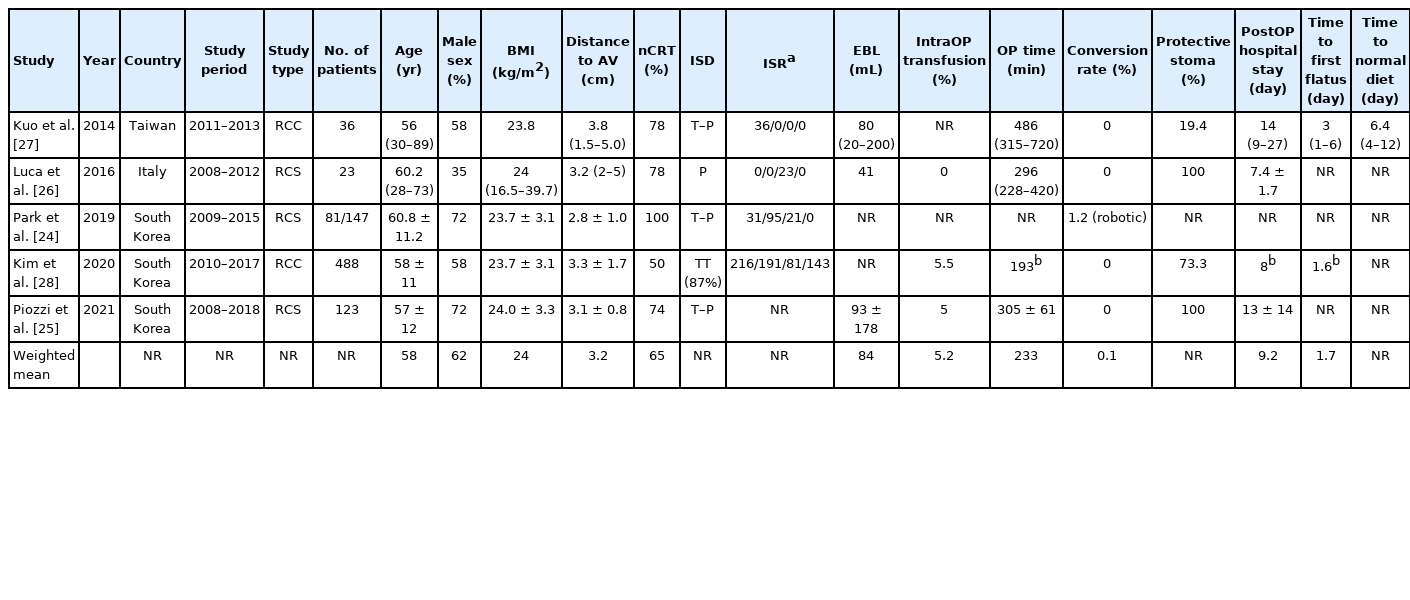
The systematic search initially identified 413 studies. After duplicates removal and screening at the title and abstract levels, 235 studies were assessed for eligibility through full-text evaluation. One study was identified from reference searching. Finally, 5 studies were included in the qualitative synthesis (Fig. 1) [24-28]. All studies were retrospective: 3 were cohort studies [24-26], while 2 were case-control studies comparing laparoscopic ISR to RISR [27] or APR to low anterior resections with or without ISR [28] (Table 1). No randomized controlled trials were retrieved. All studies were single-center experiences: 3 from South Korea, 1 from Taiwan, and 1 from Italy. The total number of patients included was 751, with the largest study performed by Kim et al. [28] (n=488). The first study was published in 2014 [27].
Patients, procedures, and pathology characteristics
Only 2 authors reported the indication criteria for RISR. Park et al. [24] have reported, in their series of post-nCRT minimally invasive ISR, that the final decision whether to perform sphincter preservation (ISR) or APR was made during restaging after nCRT. Piozzi et al. [25] reported ISR to be proposed as the first surgical strategy for patients with low-lying rectal cancers close to the sphincter complex. Infiltration of the external anal sphincter (EAS)/levator ani muscle (LAM) at the restaging magnetic resonance imaging (MRI) after nCRT and/or presence of abundant mucinous component and/or involvement of the anal canal down below the dentate line requiring total removal of the internal anal sphincter muscle (IAS) and/or preoperative documented impaired fecal continence, and/or simply patient’s refusal were considered exclusion criteria for ISR and direct indication for APR [25]. Advanced clinical T stage, even not responding to nCRT, was not considered a contraindication to ISR if curative resection was technically feasible at the preoperative MRI staging (no evidence of EAS/LAM involvement) [25].
The weighted mean age of patients submitted to RISR was 58 years with males being the majority (weighted mean, 62%; range, 35%–72%) (Table 1). Weighted mean body mass index was 24 kg/m2. The distance between the caudal edge of the tumor and the AV ranged between 2.8 and 3.8 cm (weighted mean, 3.2 cm).
All the retrieved studies reported the indications to nCRT [24-28], with 3 authors reporting also the therapeutic schemes [24-26]. nCRT was indicated for T ≥ 3, N+ low rectal tumors by 4 authors [24, 26-28]. Park et al. [24] indicated nCRT also for T2 cancers close to or involving the anal sphincter. The clinical indication for nCRT was differently described by Piozzi et al. [25]: (a) cT3/4 tumors with threatened or suspicious CRM; (b) tumor’s lowest margin involving the dentate line (regardless of T stage); (c) the presence of LNs > 5 mm in short-axis diameter on the lateral pelvis (outside the dissection plane of total mesorectal excision [TME]). nCRT ranged between 50% and 100% in the reviewed series (weighted mean, 65%). Time from nCRT completion to surgery was 6 to 7–8 weeks [24, 27], 8 to 10 weeks [25], or within 11 weeks [26].
All the authors described the surgical procedure [24-28]. Trocar placement was reported by 4 authors [25-28]. All the authors operated with a transabdominal approach; however, the intersphincteric dissection (ISD) was performed in 2 stages (transabdominal and perineal) by 3 authors [24, 25, 27], and as a single stage with a perineal approach [26] or a total transabdominal approach (in 87% of cases) [28]. The type of ISR (partial, subtotal, and total) [9, 29] was reported in all except 1 study [25]. However, Piozzi et al. [25] have included only partial and subtotal ISR because they consider patients requiring total ISR to have negative functional outcomes, therefore, indicating APR for such cases. Only Kim et al. [28] have included patients submitted to partial excision of the EAS (< 25% of muscle, n=143), performed to optimize the resection margins (CRM). All surgeries were performed with the da Vinci robotic platforms, but only 1 author specified which platform was used [25]. Piozzi et al. [25] and Leong et al. [30] described the use of a 2-stage single-docking procedure without changing the position of the patient-side surgical cart. Also, Luca et al. [26] performed a fully robotic single-docking technique. The other authors did not specify the docking strategy.
The open conversion rate was nil in all studies except for Park et al. [24] reporting a rate of 1.2% (n=1) but failing to explain the cause. Operating time was relatively variable, ranging between 187 and 486 minutes (weighted mean, 233 minutes). Kim et al. [28] reported statistical differences in operation time length between total and partial/subtotal ISR (P<0.001), with no differences between partial and subtotal ISR.
Estimated blood loss was low ranging between 41 and 93 mL with intraoperative transfusions ranging between 0% and 5%. Two authors reported a 100% rate of protective stoma creation [25, 26], while Kim et al. [28] reported a rate of 73.3%. Park et al. [24] reported that most patients received a stoma, while Kuo et al. [27] stated only 19.4%.
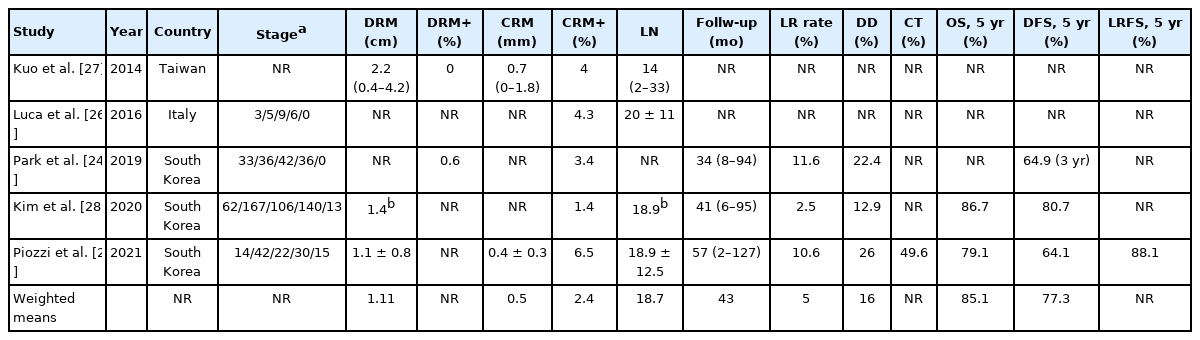
All authors except 1 [27] provided details on the cancer staging. Overall cancer staging was 112 stage 0 (14%), 250 stage I (32%), 179 stage II (23%), 212 stage III (27%), and 28 stage IV (4%) (Table 2). Mean LN retrieval ranged between 14 and 20 (weighted mean, 18.7). Three authors provided details on the DRM length (weighted mean, 1.1 cm) [25, 27, 28], while 2 provided the DRM positivity rate (0% [27] and 0.6% [24]). Two studies reported the mean CRM width (0.7 mm [27] and 0.4 mm [25]). All the authors reported the CRM rate which ranged between 1.5% and 6.5% (weighted mean, 2.4%).
Perioperative outcomes and complications
Postoperative average hospital stay ranged between 7.4 and 14 days (weighted mean, 9.2 days). Only 2 authors reported the time to first flatus ranging from 1.6 to 3 days [27, 28], with Kuo et al. [27] showing the time to a normal diet to be 6.4 days (range, 4–12 days).
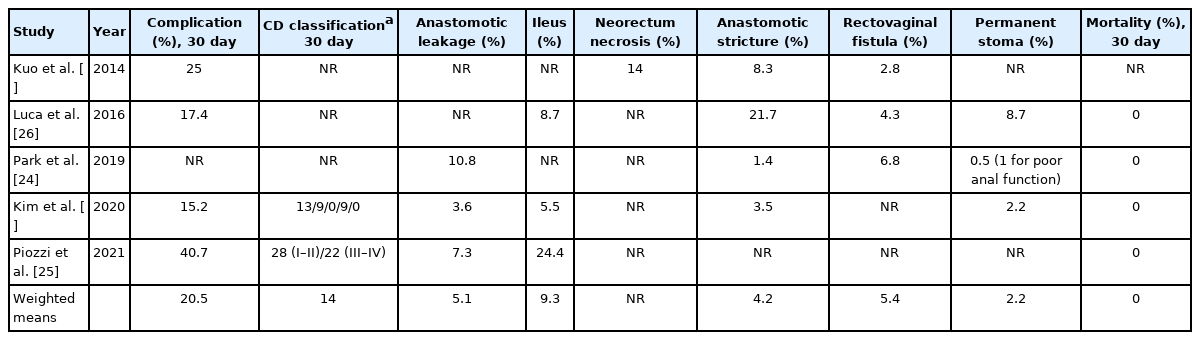
Complications rates ranged between 15.2% and 40.7% (weighted mean, 20.5%) (Table 3). Piozzi et al. [25] have reported the highest rate of perioperative complications (40.7%), which can be explained by the prospectively collected evaluation on postoperative morbidity and mortality through a weekly basis divisional quality improvement meeting. Park et al. [24] reported the complications in all the series without separating laparoscopic and robotic patients. Only 2 authors [25, 28] reported the complications according to CD classification [22]. Kim et al. [28] reported 13 grade I, 9 grade II, and 9 grade IIIb, while Piozzi et al. [25] reported 28 grade I–II and 22 grade III–IV. Anastomotic leakage ranged between 3.6% and 10.8% (weighted mean, 5.1%) [24, 25, 28], while anastomotic stricture ranged between 1.4% and 21.7% (weighted mean, 4.2%) [24, 26-28]. Postoperative ileus was reported to range 5.5% to 24.4% (weighted mean, 9.3%) [25, 26, 28]. Rectovaginal fistula rate ranged between 2.8% and 6.8% (weighted mean, 5.4%) [24, 26, 27]. Perioperative mortality was nil for all studies apart from Kuo et al. [27] who failed to report it. Permanent stoma after RISR ranged from 0.5% to 8.7% (weighted mean, 2.2%). Luca et al. [26] reported 2 patients with permanent loop ileostomy (1 for rapid systemic progression and 1 for persistence of rectovaginal fistula). Park et al. [24] reported no restoration of bowel continuity in 4 patients including 1 requiring new fecal diversion for poor anal function. Contrarily, Kim et al. [28] described that permanent stoma (2.2%) was consequent to intractable anastomotic complications and not to post reversal patient’s decision for poor anal function.
Oncological outcomes
Mean follow-up was reported by 3 authors ranging between 34 and 56.7 months with a weighted mean of 43 months. Local recurrence (LR) and systemic disease were reported by 3 studies [24, 25, 28] (Table 2). LR rate ranged between 2.5% and 11.6% with a weighted mean of 5% while systemic disease was reported between 12.9% and 26% with a weighted mean of 16%. Only 1 study reported the rate of adjuvant treatment (49.6%) [25].
Two studies reported 5-year OS (range, 79.1%–86.7%; weighted 5-year OS, 85.1%) and DFS (range, 64.1%–80.7%; weighted 5-year DFS, 77.3%) [25, 28]. Park et al. [24] reported a 3-year DFS of 64.9%.
Functional outcomes
Anorectal functional outcomes were discussed by 2 authors [26, 28]. Kim et al. [28] evaluated anorectal function in patients aged ≤ 75 years through fecal incontinence score (FIS) [31] and manometry measured at baseline and after 6 to 12 and 12 to 24 months. FIS indicates the sum of solid, liquid, gas, wearing pad, and lifestyle alteration score. At 12 and 24 months, mean scores did not differ significantly between partial and subtotal ISR (5.4±5.7 vs. 6.1±6.1, P=0.307), but were significantly higher in the total ISR subgroup (10.8±5.7) than in the other 2 subgroups (P<0.001–0.05) [28]. Moreover, solid incontinence rates were not different between patients submitted to low anterior resection without ISR and partial (P=0.602) or subtotal (P=0.062) ISR patients [28]. Kim et al. [28] reported that the mean manometry values were significantly lower 6 to 12 and 12 to 24 months after surgery than preoperatively (P<0.001–0.05), with most patients recovering continence after 12 to 24 months. Interestingly, maximal tolerance volume (P=0.314) and urge to defecate volume (P=0.88) did not differ significantly between patients submitted to subtotal or total ISR. The manometry values 12 to 24 months after surgery were significantly associated with several parameters; aging, female sex, advanced stage tumors, lower tumor location, preoperative chemoradiation, manual anastomosis, and longer operation time [28]. Partial excision of the EAS did not influence the manometry values except for mean resting pressure.
Luca et al. [26] reported the functional results of 91% of patients (n=21) according to Kirwan et al.’s incontinence score [32] measured 12 months after stoma closure: 71.4% grade I, 14.3% grade II, 4.8% grade III, 9.5% grade IV, and 0% grade V. The authors also measured the LARS score [33]: 57.1% patients with no low anterior resection syndrome (LARS, score< 20), 19.0% with minor LARS (score between 20 and 29), and 23.8% with major LARS (score > 29) [26]. No association was reported between major LARS and age, sex, clinical stage, tumor distance from the AV, presence of anal stenosis, or nCRT.
Kim et al. [28] reported a 23.3% rate of male sexual dysfunction through an institutional 4-grade scale (none, mild, moderate, and severe) measuring erectile firmness and maintenance, along with satisfaction, at baseline and 12 to 24 months after surgery. The authors also reported a total of 3.9% of patients with moderate (voiding difficulty for 2 weeks to 6 months after surgery) to severe (need for clean intermittent catheterization after 6 months) grades of urinary dysfunction according to an institutional scale.
Methodological quality
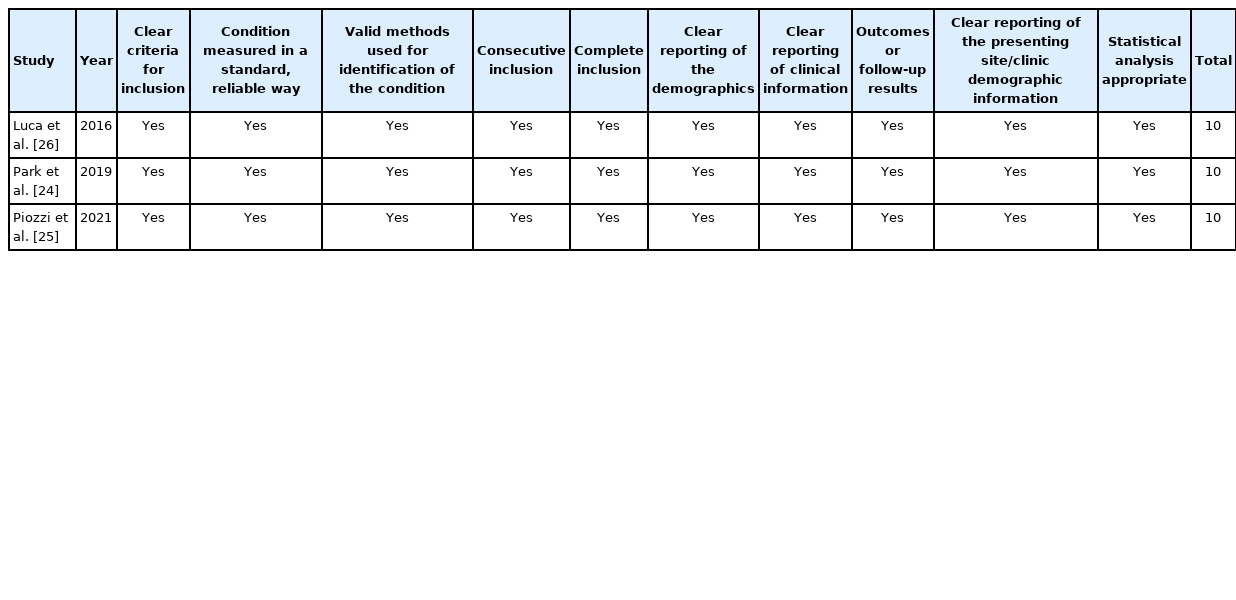
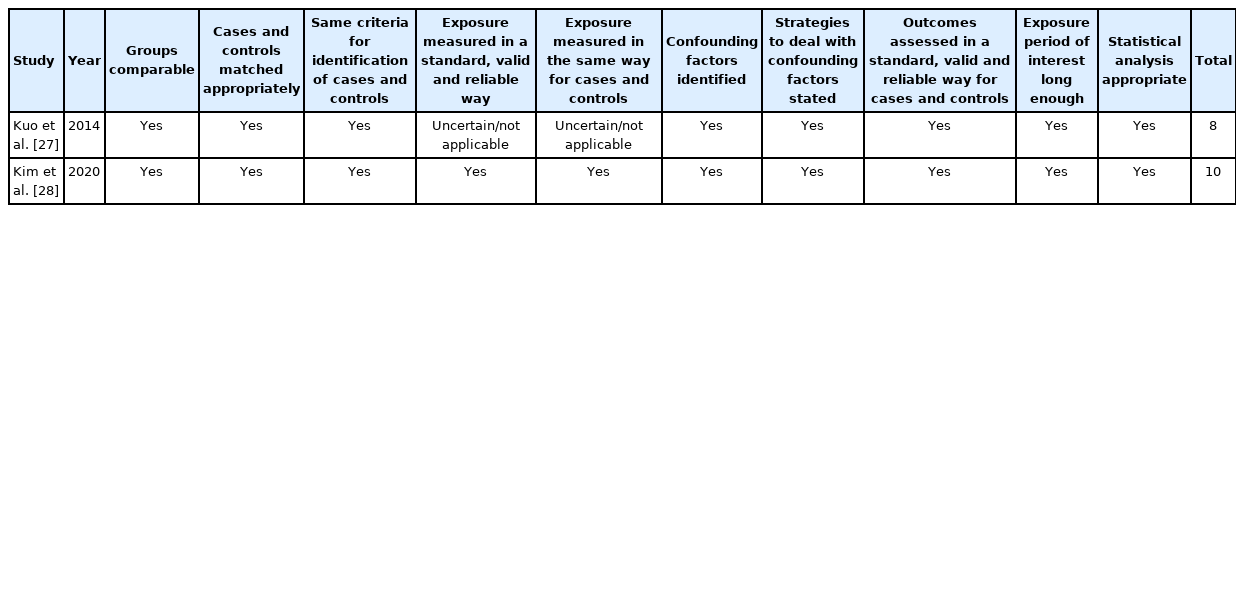
Results of the critical assessment for each study are reported in Tables 4, 5. All studies were of high quality, with 4 of them scoring full (10/10). The study of Kuo et al. [27] was of high quality (8/10) but failed to meet the criteria for Question 4 “Did the case series have consecutive inclusion of participants?” and Question 5 “Did the case series have complete inclusion of participants?”
DISCUSSION
This review provides the state-of-the-art on RISR by evaluating and discussing the results from 5 reports. Three studies were performed in South Korea in 3 referral centers for colorectal cancer with advanced expertise in robotic surgery [24, 25, 28]. Also, the senior surgeons at Korea University Anam Hospital (Professor Seon Hahn Kim), University of Ulsan College of Medicine and Asan Medical Center (Professor Jin Cheon Kim), and Kyungpook National University Medical Center (Professor GyuSeog Choi) are all surgeons with an extensive surgical practice in minimally invasive colorectal surgery (> 3,000 surgeries each).
Indications of robotic approach to intersphincteric resection
ISR is an anus-preserving technique for treating patients with low rectal cancers in alternative to APR [34]. Surgical indications have gradually changed since the first description of ISR from Schiessel et al. [1] in 1994. The widespread of TME, the standardization of nCRT protocols, and the implementation of minimally invasive approaches have further extended the clinical indications to ISR increasing the anus-preserving rate up to astonishing 98.8% as reported by Kim et al. [35] (APR rate of 1.2% in the last quarter of study period). Despite ISR has become more accepted in the last 3 decades, a consensus on the optimal clinical indications is missing and highly needed from the surgical community to standardize the technique, and the oncological, and functional results in future studies, and to implement a multicenter international ISR task force.
Generally accepted indications for ISR are T1–3 cancers, low rectal cancers (1–5 cm from the AV), and well-moderately differentiated adenocarcinoma. Contraindications are EAS/LAM infiltration, reported fecal incontinence, systemic nonresectable metastases, poorly differentiated adenocarcinoma, abundant mucinous component, psychiatric disease, and severe diseases (liver cirrhosis, cardiac failure, renal, and respiratory dysfunction) [8-10, 25].
Two recent studies, included in the review, have reported extended indications for ISR. In particular, Park et al. [24] have described that the final decision for ISR has to be rediscussed and finalized after nCRT at the restaging pelvic MRI. Tumor’s response to nCRT, MRI-based category after therapy (ymr) T stage and ymrCRM status were reported to be key factors for deciding between ISR and APR. Therefore, indications were extended to clinical stage (c) T4 patients with downstaging (i.e., ymrT0–3). The authors reported that APR should be indicated to poor responders (i.e., ymrT3) with suspicious tumor invasion of the CRM [24].
Piozzi et al. [25] further extended the ISR indications to patients with post-nCRT clearance of EAS/LAM infiltration, independently to T stage, if a curative resection was considered technically feasible. Kim et al. [28] also performed partial resection of the EAS to obtain oncologically safe CRM, extending the surgical indications for ISR. Partial resection of the EAS (with preservation of the subcutaneous sheet) was reported in case of tumors with suspected invasion into the intersphincteric space and/or EAS [29, 36, 37].
No study has yet reported the specific indications to RISR relatively to LISR or OISR. However, RISR provides optimal exposure of the narrow pelvis allowing to better detect the anatomical landmarks for an oncologically safe ISR [6, 38]. The technological improvement allows to better dissect the correct plane and in the hands of experienced surgeons also to perform a fully transabdominal approach where ISR is completely performed through the abdomen with no perineal phase.
The main limit of RISR is the cost. For example, the South Korean Health Care System does not cover the robotic approach for any type of surgery, so patients have to pay approximately an additional US $4,000 to $6,000 for choosing RISR over LISR [24]. However, approximately half of the patients are reimbursed by private insurance policies [28]. Therefore, the final indication for RISR is always determined by a joint decision between the patient and surgeon. Precise preoperative staging with the combination of rectal MRI, thoracic-abdominopelvic computed tomography scan, anal endoscopic ultrasonography, rigid proctoscopy, and digital rectal examination (DRE) remains crucial for a correct surgical indication to ISR [8]. DRE is crucial and must be performed also under anesthesia, before surgical incision, to access tumor mobility and relationship to the anal sphincters in order to proceed with ISR or convert to APR [39, 40].
Technical considerations and controversy issues
Schiessel et al. [1] described ISR as a combination of 2 techniques; the intersphincteric rectal excision for inflammatory bowel disease [41] and the coloanal anastomosis for low rectal resections [42]. According to Schiessel’s definition, ISR requires a hand-sewn coloanal anastomosis; however, some authors reported the feasibility of ISR with a stapled anastomosis [28, 43].
RISR is generally characterized by 5 steps: (1) vessel ligation (inferior mesenteric vein and artery) with left colon and complete splenic flexure mobilization; (2) pelvic dissection, (3) ISD; (4) neorectum reconstruction; and (5) ileostomy creation [30]. RISR, with both da Vinci Si and Xi platforms, requires 2 stages (left abdominal for step #1 and pelvic for steps #2–3) which can be performed with double or single-docking of the robotic cart.
Differences in (1) docking of the robotic cart, (2) role of the laparoscopic phase, (3) type of ISR approach (transabdominal and perineal, perineal only, or total transabdominal), (4) surgical steps sequence, (5) type of coloanal anastomosis, use of coloplasty, and ileostomy creation are described in the literature with no shared standardization on surgical technique allowing difficulties in comparing the oncological and functional results from different teams. Therefore, it is critical to achieving an international consensus on these steps to standardize the ISR technique.
Docking of the robotic cart
Leong et al. [30] and Kim et al. [44] have described the feasibility to perform rectal resections and ISR through a 2-stage (abdominal and pelvic) robotic approach. Avoiding a double-docking approach reduces the operation time length but requires a highly trained surgical team and a highly standardized procedure as described by Kim and Shin [44]. The trocars placement and surgical cart positioning performed in our team was described by Toh and Kim [45] and Cheong et al. [46] and are shown in Fig. 2.
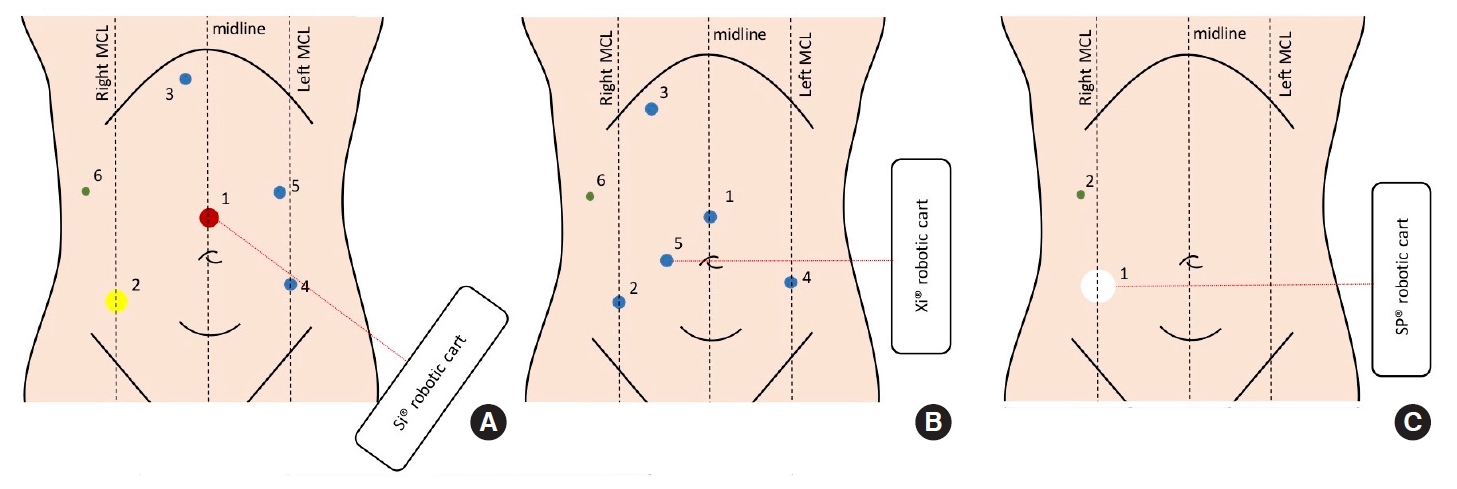
Trocar positioning for the da Vinci Si (A), Xi (B), and Single-Port (SP) as described by Toh and Kim [45] and Cheong et al. [46]. MCL, midclavicular line. Yellow circle, da Vinci 12-mm port; red circle, 12-mm standard port; blue circle, da Vinci 8-mm port; green circle, 5-mm standard port (assistant); white circle, 25-mm access for SP placement.
Role of the laparoscopic phase
RISR can be defined as fully robotic or laparoscopic-assisted. In the first case, as described by Leong et al. [30], only the abdominal cavity exploration, at the beginning of surgery and after coloanal anastomosis, and the ileostomy creation are performed laparoscopically. This technique was defined and optimized in our center and does not require changing the position of the patient-side surgical cart allowing a straightforward procedure.
In the second case, as described by Park et al. [47], also the vessel ligation (inferior mesenteric vein and artery) with left colon and complete splenic flexure mobilization is performed laparoscopically. This technique allows a single-stage (pelvic) single-docking RISR.
Controversies on approaches to interphincteric dissection
Three different types of ISD approaches have been described; transabdominal and perineal, perineal only, or total transabdominal. Each of them is characterized by pros and cons. Traditionally, according to Schiessel’s description, ISR is performed with a 2-phase ISD (i.e. transabdominal and perineal). During the abdominal phase, the intersphincteric plane is dissected as caudally as possible between the longitudinal muscle (LM) of the rectum and the EAS [6]. The ISD should proceed caudally till the anterior recognition of the rectourethralis muscle (males) or the anterior dense tissue at the end of the rectovaginal septum (females) which have to be carefully dissected [6]. Afterward, the perineal phase is performed through the circumferential dissection of the anal canal at or below the dentate line. This technique is safe and well described in the literature [24, 25, 39, 48-60]; however, it can be associated with mistakes in surgical plane alignment when the ISD is performed during the perineal phase with a high risk of plane misjudgment during the rendezvous and consequent tissue injury, especially in the lateral district. During the perineal phase, after dissecting the plane between the IAS/LM complex and the EAS, the surgical plane in the lateral district can mistakenly move dorsally to the LAM into the ischiorectal fossa, resulting in a mismatch with the abdominal plane [38].
Instead, other authors perform all the ISD through the perineal phase as the last step of the abdominal phase or first surgical step (perineal-first approach) [40, 61-68]. This technique may reduce the risks of plane misjudgment since the dissection is only cranial. However, this approach can be very unfamiliar for unexperienced surgeons because it does not provide the same view as through the transabdominal approach and does not allow safe identification of the deep pelvic landmarks for an oncologically safe ISR. In the authors’ opinion, this should not be performed without a thorough experience in transanal dissections.
A total/near-total transabdominal ISR was described by Kim et al. [35], Park et al. [47], and Huang et al. [69]. This technique allows to fully embrace the technological superiority of the robotic platform by allowing the dissection through the intersphincteric plane and then into the anal canal lumen with a so-called bird’seye view [70]. Monopolar scissors are used to perform the final opening through the IAS into the lumen of the anal canal. To help the surgeon easily identify the resection margin, an assistant guides from the perineum by gently pushing the anus with forceps holding a sponge on the tip [47]. This technique is straightforward and does not require a perineal phase, therefore, avoiding injuries following mistakes in plane lineup during the 2 surgical phases (transabdominal and perineal).
Kim et al. [28] reported a success rate of total transabdominal ISR in 87% of cases; the authors reported 13% of combined approach (transabdominal and pelvic) due to anastomotic difficulties and coloanal anastomosis. However, they failed to provide a clear explanation for switching to a combined approach.
According to Park et al. [47], transabdominal ISR should not be indicated if the rectal tumor has not significantly shrunk after nCRT and/or it is located very low and obscures the DRM from the abdominal view.
In the authors’ opinion, the current RISR technique is optimized to resect very lowlying tumors with little or no need for a perineal phase.
Controversies on surgical steps sequence: abdominal vs. perineal-first approach
Some authors have proposed an alternative surgical step sequence for ISR with a perineal-first approach [71-73]. This approach includes a circumferential incision of the anal mucosa and the IAS 1 cm distal to the lower border of the tumor followed by a 5–6 cm deep dissection of the lower rectum through a perineal approach. This technique provides some theoretical technical advantages compared to the primary abdominal approach: the early perineal exposure may help the surgeon to assess the oncological feasibility of an ISR or if a conversion to APR may be needed; it could aid the surgeon to perform the TME in the distal rectum which can be challenging in obese patients or patients with bulky tumor or a narrow pelvis, especially laparoscopically [73]. Denost et al. [74] reported in a pilot randomized trial that the perineal-first approach could improve surgical quality and reduce the risk of CRM involvement. Moreover, Kanso et al. [73] performed a retrospective matched study between abdominal and perineal-first ISR (34 vs. 51, respectively). The authors reported a significant reduction in operation time in the perineal-first approach (240 minutes vs. 269 minutes, P=0.01) with no differences in postoperative morbidity/mortality rates, CRM involvement, LR rates, and long-term survival outcomes. The authors believe the perineal-first approach could facilitate the rendezvous with the TME plane dissected laparoscopically. They explained the shorter operation time could be due to the less DRE needed to decide when to stop the laparoscopic dissection and begin the perineal approach when performing an abdominal-first approach. Further prospective studies with wider series are needed to confirm the oncologic results of this approach for patients with low rectal cancer undergoing ISR. However, the perineal-first approach does not allow abdominal exploration as the first step with a possible consequent finding of peritoneal carcinomatosis after concluding the peritoneal dissection, despite this is relatively rare in very low rectal cancer [75].
The abdominal-first approach through the robotic platform allows optimal view of the pelvic anatomical landmarks in the deep pelvis and provides effective hemostasis and traction during ISD for a meticulous dissection. The superior quality of the robotic approach, compared to the laparoscopic, could challenge the results of the studies supporting the perineal-first approach [73, 74].
Future long-term studies are needed to compare the outcomes of abdominal-first versus perineal-first RISR.
Specimen retrieval, type of coloanal anastomosis, and ileostomy
Specimen retrieval is usually transanal; however, in case of difficulty in delivering as for bulky tumor/mesorectum, the specimen can be delivered transabdominally through a minilaparotomy in the left lower quadrant after extending the trocar site laterally.
According to Schiessel’s definition, ISR requires a hand-sewn coloanal anastomosis [1]; however, some authors have extended the original definition by including a stapled coloanal anastomosis [28, 43, 70, 76, 77]. This should be carefully considered when evaluating and comparing ISR studies, as perioperative results and functional outcomes might be different [43].
Moreover, many types of hand-sewn anastomosis have been used such as colonic J-pouch, transverse coloplasty, or straight coloanal hand-sewn anastomosis, according to the surgeon’s preference. Kuo et al. [78] performed straight coloanal hand-sewn anastomosis, while our team performs side-to-end coloanal anastomosis.
There are no clear indications for ileostomy creation with some surgeons always performing it after ISR [25, 26] while others only for carefully selected patients [27, 28]. The authors consider male sex, nCRT, and low tumor height as indication criteria for diverting ileostomy. Also, intraoperative findings of unfavorable anastomosis (such as sluggish blood flow from the transected bowel end) and total/subtotal ISR are considered important criteria [70, 78].
New approach—the Single-Port robotic approach (da Vinci SP)
The da Vinci Single-Port (SP) System (Intuitive Surgical, Inc.) (SPISR) is the latest generation of robotic platforms. This platform has a revolutionary design compared to the previous multiport platforms (Si and Xi). The platform is composed of a single arm (25 mm in diameter) capable to rotate 360° on its axis allowing multiquadrant anatomical access without the need for redocking. A maximum of 3 instruments, together with the camera, are all located within the single arm and are all characterized by an additional elbow joint proximal to the wrist joint. This new joint allows triangulation and independent planar movement of the instrument tips with no risk of arm collision which was 1 of the drawbacks of the multiport platforms. Kim et al. [79] have reported the first case of SP-ISR on a 73-year-old male with a pathologic stage after theraphy (yp) IIIC rectal cancer at 5 cm from the AV. Also, Cheong et al. [46] have reported a SP-ISR on a 57-year-old female patient with an ypT2N0M0 (0/20; CRM, 7 mm) rectal cancer at 3 cm from the AV. In both cases, the SP system was located at the right lower quadrant with a single incision of around 25 to 30 mm. This position was chosen for 2 reasons: (1) to allow optimal multiquadrant view and access (splenic flexure, descending colon, and rectum); (2) to use for specimen extraction and stoma site. However, an additional trocar (12 mm) is still required because the SP platform lacks a vessel sealer, suction, clip applier, and stapler. Future studies with extended series are needed to outline the perioperative, oncological, and functional outcomes of SP-ISR.
Perioperative outcomes and complications
Conversion to open rates
Conversion to open rates ranged between 0% and 1.2% (weighted mean of 0.1%). However, Park et al. [24] failed to report the reason for open conversion in their series. A previous meta-analysis from Lee et al. [80] reported RISR to be associated with a significant decrease in conversion to open rate compared to LISR (relative risk [RR], 0.22; 95% CI, 0.05–0.97; P=0.04). The authors reported a 1.31% rate in the RISR which is higher than the weighted mean rate from the current review.
The near-nil conversion to open rate in the current study could be consequent to the high expertise of the included surgeons on robotic rectal surgery especially on anus-sparing procedures as ISR. Previously, the ROLARR trial [81] proved on subgroup analysis that surgeons with high robotic expertise (> 100 robotic operations before trial) had consistently lower odds of conversion with the robotic approach when compared to the laparoscopic (OR, 0.304; 95% CI, 0.094–0.988). This suggests a possible role of the learning curve effect of the surgeons included in the current review (surgeons with > 3,000 minimally invasive surgeries) that may have optimized the results (conversion to open rate near to nil). Moreover, the conversion to open rate is lower compared to several published LISR studies (0%–21.8%) [71, 82-84]. This indirectly shows that the robotic platform allows to safely complete an advanced anus-sparing rectal surgery, as ISR, with no need to convert to the open approach. This is crucial considering that conversion to open is a complication that is associated with disease recurrence, 30-day morbidity, and mortality [85-87]. A recent meta-analysis evaluating only randomized controlled trials and propensity score-matched studies demonstrated that rectal robotic surgery is associated with a statistically significant lower conversion to open rate compared to laparoscopy (6.7% vs. 14.5%; OR, 0.38; 95% CI, 0.30–0.46) [88].
Operation time
Operation time ranged between 193 and 486 minutes (weighted mean, 233 minutes). A previous meta-analysis from Lee et al. [80] reported a significantly longer mean operation time for RISR compared to LISR (330.3±99.3 minutes vs. 287.6±81.7 minutes, respectively; mean difference [MD], 41.89; 95% CI, 15.51–68.27; P=0.002). A multicenter study involving 7 institutions from the Korean Laparoscopic Colorectal Surgery Study Group confirmed significantly longer operation time for RISR vs. LISR (271.6 minutes vs. 232.6 minutes, P=0.001) [89]. Despite these results, the increase in robotic surgical expertise could have shortened the operation time for RISR, therefore, further new studies are needed to evaluate timing differences.
Time to first flatus
Time to first flatus ranged between 1.4 and 3 days with a weighted mean of 1.7 days. The meta-analysis of Lee et al. [80] reported the postoperative time to first flatus not to be significantly different between RISR and LISR (MD, –0.23; 95% CI, –0.75 to 0.29; P=0.38); however, the analysis was performed only on 2 studies. The authors reported a mean time to first flatus of 2.7±0.4 days after RISR which was longer than the weighted mean of the current study.
Postoperative length of hospital stay
Postoperative average length of hospital stay ranged between 7.4 and 14 days (weighted mean, 9.2 days) which is similar to that of LISR and lower than OISR [13]. A meta-analysis comparing LISR to RISR, reported a shorted length of hospital stay for RISR, but the difference was not statistically significant (MD, –0.97; 95% CI, –2.11 to 0.17; P=0.10) [80]. However, the study reported a mean postoperative hospital stay of 11.0±2.0 days in the RISR group which was longer than the weighted mean duration of the current study (9.2 days), highlighting a possible shortening of the length of hospital stay in the latest period of study due to increased expertise (study range, 2014–2021).
Complications rate
Overall complications rates ranged between 15.2% and 40.7% with a weighted mean of 20.5%. Piozzi et al. [25] have reported the highest rate of perioperative complications (40.7%) which can be explained by the high-quality prospective data collection on postoperative morbidity and mortality through a weekly basis quality improvement divisional meeting. Lee et al. [80] reported a lower overall complications rate in the RISR group (22.34%) compared to the LISR group (26.58%) but with no statistical difference (RR, 0.81; 95% CI, 0.59–1.11; P=0.19). The wide range in complications rate in the present study may be explained by the different quality of data collection between studies. Moreover, only 2 studies [25, 28] reported the complications according to CD grading system. Lee et al. [80] reported no difference in severe postoperative morbidity (CD grade ≥ III) between RISR (9.52%) and LISR (11.39%) (RR, 0.81; 95% CI, 0.48–1.34; P=0.41). In the current study, Piozzi et al. [25] reported a rate of 17.8% of CD grade≥ III, while Kim et al. [28] reported a surprisingly extremely low rate of 1.8%. Future prospective multicenter high-quality studies, designed on evaluating postoperative morbidity and mortality, are necessary to outline a more precise rate and confirm the short-term safety of RISR.
The anastomotic leak rate ranged between 3.6% and 10.8% with a weighted mean of 5.1% which was lower than the rate of 7.69% reported by Lee et al. [80]. The meta-analysis showed no significant difference in anastomotic leak rate between RISR and LISR (RR, 1.15; 95% CI, 0.61–2.19; P=0.66).
Postoperative ileus ranged between 5.5% and 24.4% with a weighted mean pool of 9.3% which was higher than the reported rate in RISR (6.6%) from Lee et al. [80]. The meta-analysis showed no significant difference in postoperative ileus rate between RISR and LISR (RR, 0.88; 95% CI, 0.41–1.92; P=0.75).
The anastomotic stricture rate ranged between 1.4% and 21.7% with a weighted mean of 4.2%. Kim et al. [70] reported that around 6.3% to 16% of ISR patients in literature have an anastomotic stricture, with nCRT, anastomotic ischemia, anastomotic dehiscence, obesity, and pelvic sepsis being possible risk factors [50, 63, 90, 91]. Cong et al. [43] reported a higher rate of anastomotic stricture in patients submitted to hand-sewn compared to stapled coloanal anastomosis (P=0.028); however, this should be confirmed by further studies.
Kuo et al. [78] reported a detailed study on the clinical outcomes of anorectal status after RISR. In this retrospective study, they specifically evaluated 3 postoperative complications; external hemorrhoids, anastomotic stenosis, and neorectal mucosal prolapse. The study was performed by prospectively collecting photographic records of perineal conditions and DRE examination up to 6 months from surgery, together with a standard follow-up oncological schedule. In their series of 108 patients, they reported 78.7% of patients experiencing edematous and painful hemorrhoids, 42.6% of anastomotic stenosis, and 14.8% of neorectal mucosal prolapse. Longer operating time was reported as a possible risk factor for external hemorrhoids (OR, 1.01; 95% CI, 1.00–1.03; P=0.043) in multivariate analysis. However, it must be noted that the OR was close to 1 so this assumption should be taken carefully. The authors postulated that prolonged application of the Lone Star rectractor (CooperSurgical, Inc.,Trumbull, CT, USA) together with hematoma formation could have contributed to hemorrhoid development. Diverting stoma was associated with earlier hemorrhoid resolution in univariate analysis postulating a possible role of stool passage as a negative factor. Leong et al. [92] have also reported a case of edematous and painful external hemorrhoids after RISR advocating that prophylactic hemorrhoidectomy should be performed during coloanal anastomosis for a patient with a history of a preexisting external hemorrhoid. Post-ISR hemorrhoid can be easily explained by the engorgement of the external hemorrhoidal plexus following the disruption of the internal hemorrhoidal plexus during ISD and transection of the middle hemorrhoidal veins during TME. ISR creates an area of relatively poor venous drainage in the anal canal causing piles. Kuo et al. [78] reported that post-ISR hemorrhoids were treated as per conventional hemorrhoids with topical mediation, warm sitz baths, and a high fiber diet. Male sex was reported as a significant risk factor for anastomotic stricture (OR, 19.1; 95% CI, 3.12–255; P=0.007) in multivariate analysis [78]. The authors believe that the narrow male pelvis could challenge a precise dissection and creation of an optimal anastomosis resulting in poorer healing and greater stenosis rate. Vascular perfusion to the anastomosis could have been impaired by nCRT as reported in the univariate analysis only. The authors reported clinical improvement in 60.4% of patients through weekly transanal dilations with Hegar dilators. In our opinion, the risk of anastomotic stenosis in ISR, as for other colorectal anastomoses, is mainly linked to impaired blood perfusion. Preoperative nCRT, extended dissection through the intersphincteric plane, the low level of the anastomosis with the consequently extended mobilization of the proximal colon may be risk factors for impaired blood perfusion to the coloanal anastomosis. Future studies addressing these issues could better outline this complication.
Kuo et al. [78] couldn’t detect any risk factors in multivariate analysis for neorectal mucosal prolapse. They postulated that a pressure difference between the anal canal and the abdominal cavity may result in an intussusception of the colon. Also, Alessa et al. [93] reported 2 cases of neorectal mucosal prolapse after RISR. A possible cause of neorectal mucosal prolapse is the complete detachment of the proximal colon from the surrounding pelvis. During ISR the rectum is not only completely detached from the LAM as for low anterior resection but also from the surrounding hiatal ligament, the EAS, and anteriorly the rectourethralis muscle (males) or the anterior area of muscular intermingling (females) [38]. The rectum is attached to the pelvic floor through a continuous smooth muscle tissue that envelopes it all around. Muro et al. [94] showed that this smooth muscle was strongly interlocked to the LAM which is responsible for pelvic floor support. This smooth muscle tissue anchors the pelvic viscera to the LAM allowing the latter to exert a lifting power on the former. The loss of these anchoring structures, with the remaining anal stump anchored only to the subcutaneous pars of the EAS (for total ISR) or also to part of the superficial EAS (for subtotal/partial ISR), may be responsible for a structural impairment of pelvic forces with no element pulling against the abdominal positive pressure. This may theoretically cause neorectal mucosal prolapse; however, it is not yet demonstrated. Therefore, ISR is always associated with an intrinsic risk of prolapse. For this reason, we always perform at least 4 deep stitches including the EAS at the cardinal points while crafting the hand-sewn coloanal anastomosis to increase the fixation of the anastomosis to the pelvic floor.
Permanent stoma after ISR ranged from 0.5% to 8.7% with a weighted mean of 2.2%. This rate is optimal considering that before ISR all patients with very lowlying rectal cancer were doomed to APR with a permanent stoma. Between the reviewed studies, Park et al. [24] are the only ones reporting a single case of fecal diversion for poor anal function after RISR. All the other cases of permanent stoma were consequent to rapid systemic progression [26], or intractable anastomotic complications (as a persistent rectovaginal fistula) [26, 28].
Oncological outcomes
The main aim of oncologic surgery is to provide tumor clearance. RISR can be considered oncologically safe. In the present study, the LR rate ranged between 2.5% and 11.6% (weighted mean, 5%) while systemic disease ranged between 12.9% and 26% (weighted mean, 16%).
Two studies reported good 5-year OS (79.1% and 86.7%), and 5-year DFS (64.1% and 80.7%) [25, 28]. Also, Park et al. [24] reported a 3-year DFS of 64.9%.
Yoo et al. [95] reported no significant differences between LISR and RISR in the 3-year OS (88.5% vs. 95.2%; P=0.174), 3-year recurrence-free survival (RFS, 75.0% vs. 76.7%; P=0.946), and 3-year local RFS (91.7% vs. 87.2%; P=0.466).
A meta-analysis [80] comparing RISR to LISR on 5 studies [27, 84, 89, 95, 96] reported similar 3-year OS (91.88% vs. 92.31%; RR, 1.00; 95% CI, 0.94–1.06; P=0.94), 3-year DFS (84.77% vs. 85.80%; RR, 1.00; 95% CI, 0.92–1.09; P=0.97), 3-year LR (7.61% vs. 5.92%; RR, 1.24; 95% CI, 0.57–2.71; P=0.59), between the 2 groups.
A multicenter study involving 7 institutions from the Korean Laparoscopic Colorectal Surgery Study Group [89] has analyzed a relatively large homogenous population (n=334) comparing RISR (n=163) with LISR (n=171). The authors reported similar 5-year OS, DFS, and LR between the 2 groups. No difference was shown on 3-year DFS (76% vs. 79%, P=0.887) and 3-year LR (9% vs. 8%, P=0.930) in subgroup analysis evaluating only locally advanced rectal tumors (cT3/4).
Kim et al. [15] reported a study comparing RISR to OISR for low rectal cancer. The authors showed no statistical difference in 3-year LR (3.6% vs. 3.8%, P=0.96), 3-year systemic recurrence rate (17% vs. 14.4%, P=0.58), 3-year OS (91.1% vs. 90.4%, P=0.89), and 3-year DFS (79.5% vs. 79.8%, P=0.67).
Several authors have confirmed the oncological safety of ISR compared to standard APR with reported long-term LR rate, local RFS, OS, and DFS [2-5]. Moreover, hospital stay and postoperative morbidity resulted significantly lower after ISR in a recent meta-analysis [5]. ISR is an oncologically safe anus-preserving alternative to APR and is associated with lower morbidities due to smaller perineal incision.
Functional outcomes
The advances in surgical oncology have led to an increasing trend toward anus-preserving procedures for the treatment of low rectal cancer. ISR is considered the ultimate anus-preserving technique. The partial/total removal of the IAS and the dissection through the intersphincteric plane can result in severe bowel dysfunction affecting the quality of life [74]. After confirming the oncological safety of ISR, recent interest has grown regarding functional outcomes after ISR [97, 98]. However, studies on functional outcomes are still limited in number and quality due to several limitations: (1) no standardization on functional tests and questionnaires [99]; (2) scarce use of anorectal manometry and poor description of the parameters; (3) limited analysis between total, subtotal, and partial ISR; (4) combination of stapled and handsewn coloanal anastomosis. Also, functional outcomes should take into consideration the cultural backgrounds of different populations.
Two factors should be considered when evaluating the functional outcomes of ISR; anorectal function and genitourinary function.
Luca et al. [26] have specifically evaluated the functional results of robotic total ISR with hand-sewn coloanal anastomosis (78.3% post nCRT). Continence at 12 months follow-up was measured using Kirwan et al.’s score [32], while the LARS was assessed through the LARS score [33]. The authors reported good fecal continence (Kirwan’s grade 1 and 2) in 85.7% of the patients; however, the major LARS rate was 23.8%. Despite the high rate of nCRT, no statistically significant association was reported between nCRT and major LARS; however, the series was limited in numbers.
Kim et al. [28] evaluated anorectal function in patients aged ≤ 75 years through FIS [31] and manometry measured at baseline and after 6 to 12 and 12 to 24 months. Interestingly, the mean FIS scores did not differ between partial and subtotal ISR but between total and partial/subtotal at 12 and 24 months. This difference shows that there is no difference in partial/near-total IAS excision but there is a significant functional worsening after complete excision of the IAS (P<0.001–0.05). This was confirmed by evaluating solid incontinence which was similar between patients submitted to low anterior resection with partial/subtotal ISR or without ISR. Therefore, complete removal of the IAS has clinical significance. Despite ISR was associated with a significant decrease of mean manometry values, continence was recovered by most patients after 12 to 24 months of follow-up. Interestingly, no difference was reported in subtotal/total ISR regarding maximal tolerance volume (P=0.314) and urge to defecate volume (P=0.88) confirming the relevance in preserving as possible the dentate line, as routinely performed in our center. Partial excision of the EAS did not influence the manometry values except for mean resting pressure, which is dependent mainly on the IAS. Risk factors for low manometry values at 12 to 24 months were aging, female sex, advanced stage tumors, lower tumor location, nCRT, manual anastomosis, and longer operation time [28]. However, it must be taken into consideration that the authors have reported together both stapled (end-to-end) and hand-sewn coloanal anastomosis (without any coloplasty) failing to report the rate of each technique. This limitation could be responsible for bias in the functional results.
A retrospective Korean multicenter study comparing RISR to LISR reported no significant difference in bowel function (> 10 active bowel/day, need to wear pads, need of antidiarrheal medication) between the 2 groups after a minimum of 24 months follow-up [89].
Denost et al. [74] retrospectively reported their 25 years’ experience on ISR. They reported a median LARS score of 30 (range, 0–42) with 42% experiencing major LARS, and median Wexner continence score of 9 (range, 0–20) with 44% experiencing major incontinence. A total of 12% of patients required a definitive stoma following functional disorders. The authors reported an improvement in functional results in the last period of study (2007–2014) following the introduction of an institutional bowel rehabilitation program.
Denost et al. [74] have previously reported that tumor height of > 1 cm from the anal ring (OR, 5.88, 95% CI, 1.75–19.8; P=0.004) and coloanal anastomosis higher than 2 cm above AV (OR, 6.59; 95% CI, 1.12–38.67; P=0.037) were the only independent predictors of good continence after ISR in multivariate analysis. Interestingly, patient characteristics, nCRT, surgical approach, colonic pouch use, total vs. partial ISR, specimen extraction site, and pelvic sepsis were not risk factors.
Besides oncological clearance, postoperative bowel function is the main aspect that must be critically discussed with the patient to decide whether for ISR with a possible high rate of LARS (after total ISR) or APR with a permanent colostomy. Together with preoperative fecal incontinence evaluation, postoperative quality of life must be thoroughly discussed and explored with the patient.
ISR affects genitourinary function due to the extreme extent of dissection especially in the anterior district posteriorly to the rectourethralis muscle where there is a high risk to injure the cavernous muscle inducing erectile dysfunction [100]. Kim et al. [28] reported a total of 23.3% of male sexual dysfunction by measuring erectile firmness, maintenance, and satisfaction through an institutional scale. Also, the voiding function was reported through an institutional scale with a total of 3.9% of patients with moderate to severe grades. Interestingly, patients who submitted or not to ISR had no significant difference in genitourinary function. However, both genitourinary functions were measured with systems less sensitive than the International Prostate Symptom Score or the International Index of Erectile Function. A retrospective Korean multicenter study comparing RISR to LISR reported a lower incidence of retrograde ejaculation in favor of the former but no statistical significance was reached (12% vs. 20%, P=0.124) [89].
Future high-quality multicenter studies with a standardized collection of functional outcomes through international scores are needed. Also, standardized use of anorectal manometry should be used to better describe postoperative results. Mechanical and hand-sewn coloanal techniques should be analyzed separately and according to the extent of ISR (partial, subtotal, or total).
Limitations
This study is the first systematic review on robotic ISR. This review aims to provide the current state-of-the-art and to draw considerations on low rectal cancer treatment through RISR. However, there are some limitations. There are few published reports on RISR, and they are mainly retrospective single-center studies from East Asian countries; therefore, there could be a difference in results compared to western surgical series. Also, retrospective cohort studies represent a low quality of evidence. A meta-analysis was not possible for the several differences between the studies as aforementioned. However, the authors believe that this study shows the surgical advantages of RISR which encourages future multicenter prospective studies to better analyze each aspect.
CONCLUSION
ISR is the ultimate anus-sparing technique for low rectal cancer. Thorough anatomical knowledge is crucial for a precise ISR. Indications for RISR are not yet defined. RISR is feasible with all da Vinci platforms (Si, Xi, and SP). The RISR approach and technique are performed differently by different surgical teams. Postoperative morbidities after RISR are acceptable. Postoperative external hemorrhoids, anastomotic stenosis, and neorectal mucosal prolapse should be carefully controlled. RISR allows adequate surgical margins and is an oncologically safe anus-preserving alternative to APR. RISR may result in severe bowel and genitourinary dysfunction affecting the quality of life in a portion of patients. Preoperative fecal incontinence evaluation and postoperative quality of life must be thoroughly discussed and explored with the patient for a correct surgical indication.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.