Transanal total mesorectal excision for rectal cancer: it’s come a long way and here to stay
Article information
Abstract
Transanal total mesorectal excision (TaTME) was introduced as a novel technique to deal with rectal cancers. Its transanal approach offered the shortest distance to approach a challenging location, allowing an excellent visualization of the distal resection margin. Since its introduction in 2010, a significant amount of research has been put in to measure its development. In this review, we look at its ancestry, the genesis for its introduction and continued evolution as well as some of the important outcomes in its journey thus far. The importance of a structured and proctored learning journey is also stressed to enable the safe application and development of this technique. Beyond this, the TaTME movement has progressed relentlessly and its utility has been expanded to the management of benign conditions such as inflammatory bowel disease, Hartman reversals, and anastomotic strictures. We believe that the continued development and adoption of TaTME worldwide is here to stay.
BACKGROUND
“Carcinomas of the rectum are tabooed by all practical surgeons on account of their anatomical inaccessibility. All are abandoned without hope to linger on for a few months until death relieves them of their loathsome condition.”
-Maunsell HW
Management of rectal cancer has thankfully evolved significantly since those dark days. At present, it is treated in a multimodal fashion with ever-increasing use of effective neoadjuvant and adjuvant treatments; which includes chemotherapy and radiotherapy. Proper oncological surgical excision however remains the cornerstone of treatment. Since introduction of the concept of total mesorectal excision (TME) by Heald et al. [1] in 1982, it has rapidly gained traction and is now the gold standard surgical technique for curative resections [1]. Indeed, when performed satisfactorily with negative margins, TME excision is perhaps the single most important predictor of local recurrence with a corresponding improvement in disease-free survival [2].
The 1990s saw the advent of laparoscopic surgery and it soon made its way to rectal oncological work. There is little doubt that a laparoscopic approach to TME has improved intraoperative visibility significantly compared to the open approach. Results from the CLASICC (Conventional versus Laparoscopic-Assisted Surgery In patients with Colorectal Cancer) and COLOR II (COlorectal cancer Laparoscopic or Open Resection II) trials further support the use of laparoscopic surgery with improved short-term patient outcomes and minimal difference in oncological outcomes [3, 4]. The laparoscopic resection of rectal cancer, particularly in a narrow and deep pelvis is however technically challenging and often requires a prolonged training curve. Commonly cited technical limitations include the rigidity of straight laparoscopic instruments, unstable camera ship, and unsatisfactory ergonomics for the operating surgeon [5].
Robotic assisted surgery (da Vinci Surgical System, Intuitive Surgical, Sunnyvale, CA, USA) was introduced in 2000 and quickly promoted as a means to overcome some of these issues. The nature of rectal surgery with its difficult ergonomics appear well suited to a robotic approach. The articulated instruments, 3-dimensional camera view, and a stable camera platform confer significant advantages to the operating surgeon and should result in finer dissection in the confines of the pelvis [6, 7].
A full robotic approach is however not all rosy. The 2 operative fields (splenic flexure takedown and rectal dissection), potential collisions of robotic arms, lack of tactile feedback and longer operating times are all reported disadvantages of the system. In addition, ROLARR (Robotic vs Laparoscopic Resection for Rectal Cancer) trial also suggests no statistically significant evidence of proposed superiority compared to laparoscopic surgery [8]. However, we must bear in mind that the robotic platform is constantly evolving. Innovative designs such as the use of “smart” staplers, energy device incorporation, and computer-assisted intelligence have already improved the robotic operative experience and will certainly have the potential to further shape the future of surgery [9].
The subsequent development of transanal TME (TaTME) was therefore motivated primarily by all these difficulties surrounding lower rectal dissection. This transanal approach simply offered the shortest distance to approach an otherwise tricky location, allowing an excellent visualization of an adequate distal resection margin in the process.
It is useful at this point to note the key difference between the various modalities discussed. Laparoscopy and robotic assisted surgery are both tools used in the conduct of transabdominal pelvic surgery. This basically means all anatomical concerns that surgeons face in the abdomen and pelvis will continue to persist: a narrow pelvis, an obese patient, and a very distal rectal tumor with uncertain distal transection margins. TaTME on the other hand is an approach, an approach which revolutionizes the way we look at a similar problem. It is thus impossible for us to perform a head-to-head comparison between all 3 modalities on every front.
ANCESTRY OF TRANSANAL TOTAL MESORECTAL EXCISION
“Transanal TME is a new solution to some old problems.”
-Heald RJ
TaTME can really be attributed to 3 key phases in the evolution of rectal cancer management: (1) Dr. Richard J. Heald’s landmark description of the TME plane in 1982, which quickly became a cornerstone of rectal cancer surgery [1]; (2) Dr. Gerhard Buess’s invention of the transanal endoscopic microsurgery (TEMS) platform to allow transanal excision of rectal polyps and early cancers [10]; (3) In 1996, Dr. Gerald Marks championed the notion of giving preoperative radiotherapy for rectal cancer downstaging to increase sphincter preservation. This was followed by transabdominal transanal (TATA) radical proctosigmoidectomy with a coloanal anastomosis as a means to restore bowel continuity and avoid a permanent colostomy [11].
These 3 crucial phases in rectal cancer surgical development essentially created a fertile environment for modern-day TaTME to develop. Other innovative minimally invasive surgery approaches soon followed, all adding to the development of TaTME. Single-incision laparoscopic surgery was developed by Castellucci et al. [12] in 2008 and made its way to colectomies in the same year. Natural orifice transluminal endoscopic surgery (NOTES) emerged and an ever-increasing variety of procedures were completed smoothly in both animal models and clinical patients [13].
The real driver behind the development of TaTME perhaps lies in the simple fact that good rectal cancer surgery is really not easy. There are many considerations: negative resection margins particularly distally, sparing of the sphincter, nerves, and other vital structures and finally performing a safe anastomosis of the neorectum to the distal rectum or anus. All these, while within the limited confines of the deep and potentially narrow pelvis. Surgeons were therefore looking eagerly for a new breakthrough technique.
It came in 2010. Transanal minimally invasive surgery (TAMIS) was introduced that year by Albert et al. [14] as a feasible alternative to TEMS [14]. It proved to be a lot more. The lower cost, flexible, and laparoscopic-based platform which is a lot more intuitive to many modern-day surgeons, proved to be warmly received and resulted in the TaTME movement. The same year saw the first series of 20 patients undergo TaTME successfully by de Lacy et al. [15]. Before long, many other subsequent studies also demonstrated similar successes, proving the safety and feasibility of this innovative “bottom-up” approach [16]. This heralded the evolution of modern-day TaTME as we know it.
OUTCOMES
“If I have seen further than others, it is by standing upon the shoulders of giants.”
-Isaac Newton
Since its inception in 2010, a significant amount of time, effort, and research has been dedicated to gauging its development. The TaTME approach particularly with a “Cecil” approach, incorporating 2 surgical teams has been shown to reduce surgical time significantly with both Chen et al. [17] and Thien et al. [18] reporting up to a 60 minutes reduction in operative time. The direct visualization of the tumor on entry has also brought relief to many surgeons. There were concerns raised though.
The initial concerns quite intuitively were mainly focused around the quality of TME specimen and its ability to consistently deliver good oncological outcomes. Other relevant questions included the ability to minimize pelvic sepsis and of course cancer seeding, having divided bowel from the onset in this technique. Its results can largely be summarized into perioperative outcomes, oncological and functional results.
Perioperative outcomes
TaTME has given surgeons a refreshing opportunity to perform a “down to up” approach. While yielding better visibility coupled with other advantages, this change in anatomical perspective has also created unique complications including the now infamous urethral injury. The limited confines surgeons have to operate in, new surgical planes from a different viewpoint and issues with pneumodissection have misled even the most experienced rectal surgeons. In a systematic review by Bjørn and Perdawood [19], notable intraoperative complications while not common, included pneumatosis of the small bowel mesentery, intraoperative bowel perforation, and urethral injuries during transanal dissection and a single case of air embolism.
Urethral injury
Injury to the membranous portion of the urethra during the initial transanal dissection phase is often a direct result of unfamiliarity with surgical planes, particularly in the male patient. While relatively uncommon today, it is a troublesome complication to address, particularly since most patients have received preoperative radiotherapy with expected impaired tissue healing abilities. Sylla et al. [20] reported in an international collaborative retrospective study an incidence of 1.9% (34 urethra injuries out of 1,833 TaTME cases). They further mentioned in their report that the majority of the injuries occurred within the first 8 cases of the respective surgical teams’ journey. We firmly believe this can be circumvented with a structured training with a proctor for the initial learning phase.
Anastomotic leak
Anastomotic leaks are the bane of rectal cancer surgery. Perhaps the most tangible advantage of TaTME is the clean and precise distal rectal transection at the start of surgery as opposed to the repeated staple fires in both laparoscopic and robotic assisted surgeries. This allows a single stapler technique to be employed in a vast majority of cases, with proponents championing a lower leak rate. Unfortunately, studies have come up with mixed data and have failed to consistently portray TaTME as a superior option in this aspect.
Ma et al. [21], in their systematic review comparing TaTME with laparoscopic TME found comparable anastomotic leak rates (odds ratio [OR], 0.78; 95% confidence interval [CI], 0.44–1.40; P=0.41) [21]. Spinelli et al. [22] in 2021 on the other hand reported very encouraging results of vastly reduced leak rates; 17.5% in the conventional laparoscopic double stapled group vs. 6% in the TaTME, single stapled group (P=0.005). It must be noted however that the majority of these results were from early experiences with limited case numbers.
We know anastomotic leak is a multifactorial complication, influenced just as much during transection as anastomosis. The authors believe that with proper patient selection, structured training, and eventually proctored clinical application, TaTME with its single stapling technique can certainly be comparable if not superior in terms of anastomotic leak rates. And with a burgeoning group of mature TaTME surgeons worldwide and a methodical anastomotic technique, we might well be seeing divergent results in the future.
Oncological results
TaTME was developed primarily to achieve better TME dissection with more accurate visualization of tumor margin. Dr. de Lacy’s group [23] further reinforced this belief by reporting a high TME quality in their experience of 186 patients. TME was found to be complete or near-complete in 97.3% of the patients with reported circumferential and distal margin positivity of 8.1% and 3.2%, respectively. A systemic review of Ma et al. [21], which compared TaTME with laparoscopic TME, also showed a significantly higher quality of TME specimens in the TaTME group (OR, 1.75; 95% CI, 1.02–3.01; P=0.04).
These pathological advantages have not, however, always translated to our ultimate goals of lower recurrence rate and prolonged long-term survival. The Norwegian moratorium on TaTME in 2019 singlehandedly led to a temporary ban on TaTME in that region. In that paper, Larsen et al. [24] reported a whopping 9.5% local recurrence rate. Even more worrying was the early median time to recurrence of 11 months and the multifocal pattern of local recurrence.
However, we believe that even though there are obvious aspects of TaTME that can hypothetically cause tumor spillage; such as a suboptimal initial oncological purse string, we have to bear in mind that TaTME was still evolving then and key steps have already been introduced to mitigate them. These include an airtight purse string with a thorough rectal washout. Just like how surgeons were concerned about port-site metastases when laparoscopic surgery was first initiated for colorectal cancer, surgical outcomes are after all intimately related to surgical technique and the surgeon [25].
Since then, there has been a lot more data on both short- and moderate-term oncological outcomes which suggests that TaTME is as safe as laparoscopic TME and other approaches [26-28] (Table 1 [21, 26, 29-31]). Roodbeen et al. [32] for instance reported a local recurrence rate of only 3% in their 2021 multicenter observational study of 767 patients. In addition, none of the patients developed a multifocal pattern of recurrence. While heartening, this clearly illustrates the fact that this technique should be performed in a high-volume center with dedicated proctoring in the early phases of the learning curve for it to be oncologically safe.
Functional results
Bladder, anorectal, and sexual function rank as the 3 most crucial elements of quality of life after rectal surgery. Many including even Dr. Heald himself, believe that once properly mastered, TaTME with its superior visibility of the pillars, plexuses, and neurovascular bundles during dissection can surely provide a better return to the bladder and sexual function. TaTME may however hamper anorectal function with a possible lower anatomical anastomosis and the transanal platform placing an intraoperative stretch on the anal sphincters further aggravating the problem.
Koedam et al. [33] objectively reported their midterm findings on the quality of life after rectal surgery. They found that even though the quality of life which included bladder, sexual, and anorectal significantly reduced postoperatively, most returned to baseline 6 months after surgery. Their study of 30 consecutive TaTME patients also found a low anterior resection (LAR) syndrome of 33% after stoma reversal, comparable to laparoscopic TME rates. Another study of 54 consecutive patients compared 27 TaTME patients to 27 laparoscopic resection ones [34]. They found comparable outcomes in quality of life between both groups with no significant differences in terms of LAR syndrome or bladder function.
A multicenter prospective observational study comparing 55 robotic TME and 65 TaTME also reported similar sexual functions in both groups. The robotic group however reported slightly better anorectal function with the TaTME group superior in terms of preserving male urinary function [29]. The dearth of high-level evidence and limited studies makes it challenging to comment on the functional results of TaTME and we have to await further high-quality evidence such as the ongoing COLOR-III trial to shed more light on this issue [35].
MOUNTING THE CURVE
“Give me 6 hours to chop down a tree and I will spend the first 4 sharpening the axe.”
-Abraham Lincoln
The common theme while evaluating the outcomes of TaTME is an overwhelming need for a structured training program and proctorship during the initial phases of clinical application. As with all new and challenging surgical techniques, the proper evolution of TaTME into a reproducible surgical technique with standardized steps and identification of key anatomical landmarks with consistent results has taken time.
In our experience, we favor the 2-team synchronous approach which allows coordinated retraction of the rectum and a significant reduction in operative time [17]. We also use a flexible transanal platform (Gel-POINT Path, Applied Medical, Rancho Santa Margarita, CA, USA) (Fig. 1A). The initial purse string, which we term the “oncological” purse string is crucial in determining the distal margin and sets the stage for subsequent dissection into the TME plane (Fig. 1B). We perform an early distal washout with betadine thereafter to minimize tumor cell spillage and colonic contamination. Once circumferential full-thickness proctectomy has been performed, we leave the anterolateral areas to the end often resulting in a “winged” appearance with subsequent dissection at the apex of the neurovascular bundle (Fig. 1C) before eventual rendezvous with the abdominal team (Fig. 1D). A running purse string, which we call the “functional” purse string is then performed either laparoscopically or hand-sewn depending on the location facilitating the entry of a transanal circular stapler and anastomosis (Fig. 1E).
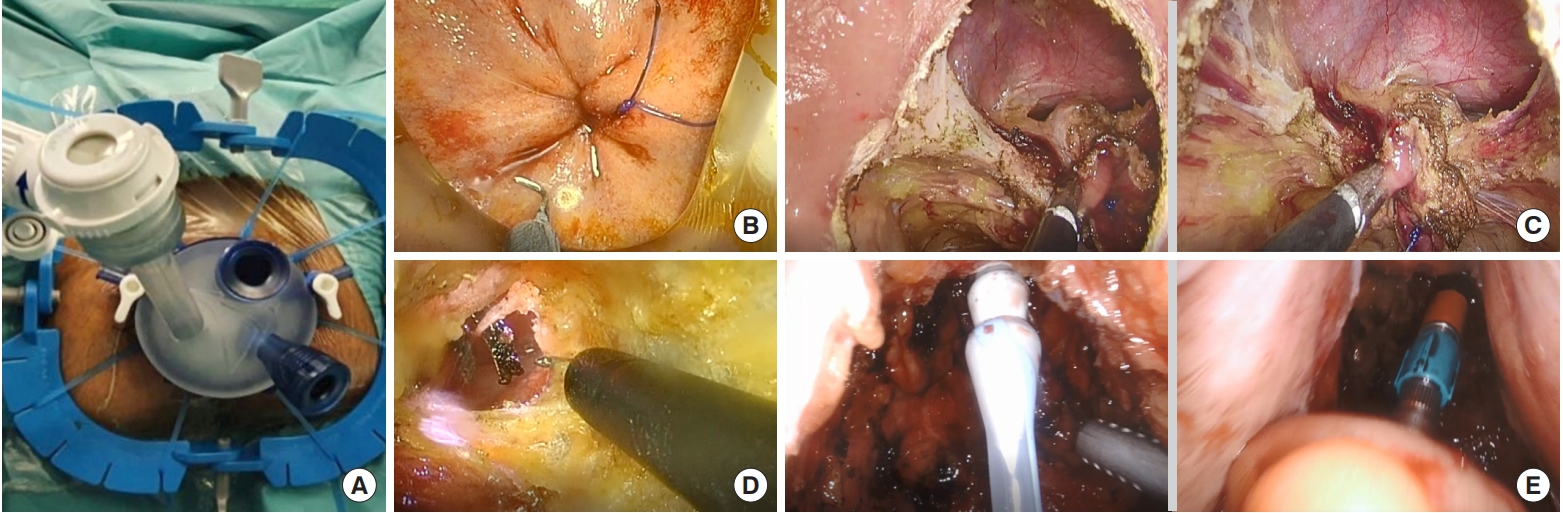
Operative procedure. (A) The setting of transanal Gel-POINT platform (Gel-POINT Path, Applied Medical, Rancho Santa Margarita, CA, USA) with Lone Star Retractor (Lone Star Retractor System, Cooper Surgical Inc., Trumbull, CT, USA). (B) “Oncological” purse string. (C) Dissection of “winged” appearance of neurovascular bundle. (D) Rendezvous procedure. (E) Introduction of circular stapler and colorectal anastomosis.
There is now consensus on a structured training program for TaTME [36]. Firstly, TaTME should not be offered in every colorectal unit but centralized to specialist centers with a minimum of at least 20 cases per year. A majority verdict was also reached that the top 2 prerequisites to commencing TaTME were completion of advanced laparoscopic training and a minimum of 30 rectal cases performed independently. In addition, a minimum of 5 TEMS or TAMIS cases was proposed as a prerequisite to attempting TaTME. The desirable prerequisites for a trainer for TaTME were at least 30 cases performed independently, provision of training courses with possible cadaveric resources and an academic output of at least 2 papers a year. This was validated in a recent paper by Ho et al. [37], where they reported safe perioperative and oncological outcomes in their medium-sized colorectal unit of around 30 rectal cancer resections a year.
Perhaps the most important is direct clinical proctorship with regular evaluation of pathological quality of resected TME specimens and analysis of clinical outcome data including complications, mortality, and oncological results. With a robust and detailed framework for a structured TaTME training program in place, it can only serve to protect the interests of both patients and surgeons, while providing a platform for quality assurance and academic pursuit.
Beyond the learning curve, the TaTME movement progresses unabated. The combined use of both robotic assisted surgery and TaTME has already been described [38]. Robotic TaTME has also been introduced as a novel technique, incorporating both the benefits of TaTME and use of robotic technology [39]. This can potentially reduce surgical time, shorten the technical learning curve required and also reduce surgeon fatigue, synonymous with long and complex rectal cases [40]. To add to that, transperineal abdominoperineal resection, which involves a concurrent abdominal and perineal team has also been performed in numerous centers worldwide, reporting feasibility, an acceptable perioperative morbidity, and a relatively low rate of perineal wound dehiscence [41]. Further innovation, evolving technology, and experience on standardized techniques will only pave the way for future success and expansion of the TaTME movement.
CONCLUSION
What is certain, is that the continued development and adoption of TaTME, in the treatment of rectal cancer is here to stay. This is simply because the logic of commencing the most difficult part of the surgery early, starting with transanal to ensure an optimal distal margin is too obvious. The use of TaTME is also not merely confined to rectal cancer surgery. This novel “bottom-up” technique can easily be extended to other disease pathologies as well. Reversal of Hartmann’s procedure, revision of anastomotic strictures, or inflammatory bowel disease surgical management are some of the notable conditions which can benefit from this surgical technique.
The divergent outcomes of this surgical procedure should however warn young surgeons about the perils of adopting this demanding technique on their own. And while we eagerly await the final results of landmark randomized trials like COLOR-III and GRECCAR 11 for TaTME to formally seal its place in the armamentarium of proven rectal surgical techniques [35, 42], it will ultimately still fall on the shoulders of our current experienced minimally invasive rectal cancer surgeons to define limits and the ideal utilization of this very useful “bottom-up” surgical approach.
Notes
CONFLICT OF INTEREST
No potential conflicts of interest relevant to this article were reported.
FUNDING
None.

