Detection of Polyps After Resection of Colorectal Cancer
Article information
Abstract
Purpose
Because colonoscopy after colorectal cancer surgery is important for detecting synchronous or metachronous colorectal neoplasms, we designed this study to investigate, by using postoperative colonoscopy, the miss rate for and the location of polyps remaining after colorectal cancer surgery.
Methods
In a prospectively-collected patient database, 264 patients were shown to have undergone a colorectal cancer resection between May 2012 and June 2013. Of these, 116 who had received a complete colonoscopy preoperatively and postoperatively were included in this study.
Results
Of these 116 patients, 68 were males and 48 were females; their mean age was 63 years. The mean time after surgery at which postoperative colonoscopy was performed was 7.1 months (range, 3-15 months). On postoperative colonoscopy, a total of 125 polyps were detected. Of these, there were no cancerous lesions; 46 (36.8%) were neoplastic polyps, and 79 (63.2%) were nonneoplastic polyps. Fifty-nine polyps (47.2%) and 15 polyps (12%) were located in the proximal and the distal parts of the anastomosis, respectively. The miss rates for the total numbers of polyps and of neoplastic polyps remaining after surgery were 37.4% and 24.2%, respectively. The incidence of neoplastic polyps increased during postoperative colonoscopy as it had during preoperative colonoscopy (r = 0.164, P = 0.048).
Conclusion
Colonoscopic surveillance after colorectal cancer resection results in the detection of pathologic polyps in one-fourth of the cases. During postoperative colonoscopy, careful examination of the proximal colon is necessary. Patients in whom multiple neoplastic polyps had been detected during preoperative colonoscopy require careful and thorough follow-up.
INTRODUCTION
In colorectal cancer patients, preoperative and postoperative colonoscopies are essential for detecting synchronous and metachronous colorectal neoplasms. In general, the incidence of metachronous colorectal cancer is reported to be between 1.1% and 3.6% [123], and in patients with colorectal cancer, the incidence of neoplastic polyps found during postoperative colonoscopic surveillance is reported to be about 17% to 30% [45]. The National Comprehensive Cancer Network (NCCN) guidelines recommend postoperative colonoscopic surveillance to detect colorectal polyps during the first year following surgery [6].
Because most polyps detected during preoperative colonoscopy are removed before radical surgery, whether those detected during postoperative colonoscopy within one year of the surgery are polyps that were missed during the preoperative colonoscopy or metachronous lesions is not know. Furthermore, the incidence of remaining polyps is not well known, and the locations, distributions, and clinicopathologic features of these remaining lesions are not clear. For these reasons, in this research, we estimated the incidence and the distribution of the polyps, both neoplastic and nonneoplastic, remaining after a curative colorectal resection in patients with colorectal.
METHODS
All data were extracted from a prospectively collected colorectal cancer patients' database. Of the 264 patients who underwent a curative resection for colorectal cancer between May 2012 and June 2013, 116 who had undergone complete preoperative and postoperative colonoscopies were enrolled in this study. Patients were excluded based on the following criteria: patients in whom the colonoscope could not pass through the colorectal lesion due to a luminal obstruction, patients who had undergone preoperative concurrent chemoradiation therapy, patients who had undergone palliative surgery, and the patients who had undergone postoperative colonoscopy at other clinics. In the 116 enrolled patients, all polyps detected during preoperative colonoscopy had been removed. The colon was defined as being from the cecum to the rectosigmoid junction. The right colon was defined as being from the cecum to the transverse colon, and the left colon was defined as being from the splenic flexure to the rectosigmoid junction.
The miss rate of polyps was calculated as follows [4]: total number of missed polyps/(total number of missed polyps + total number of polyps detected on the initial colonoscopy).
Data analysis was performed using the SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). Summary statistics using the Student t-test with Welch correction, the Mann-Whitney test, and the chi-square test were used to compare the number of polyps according to their locations and sizes. Statistical significance was set at a P-value less than 0.05.
RESULTS
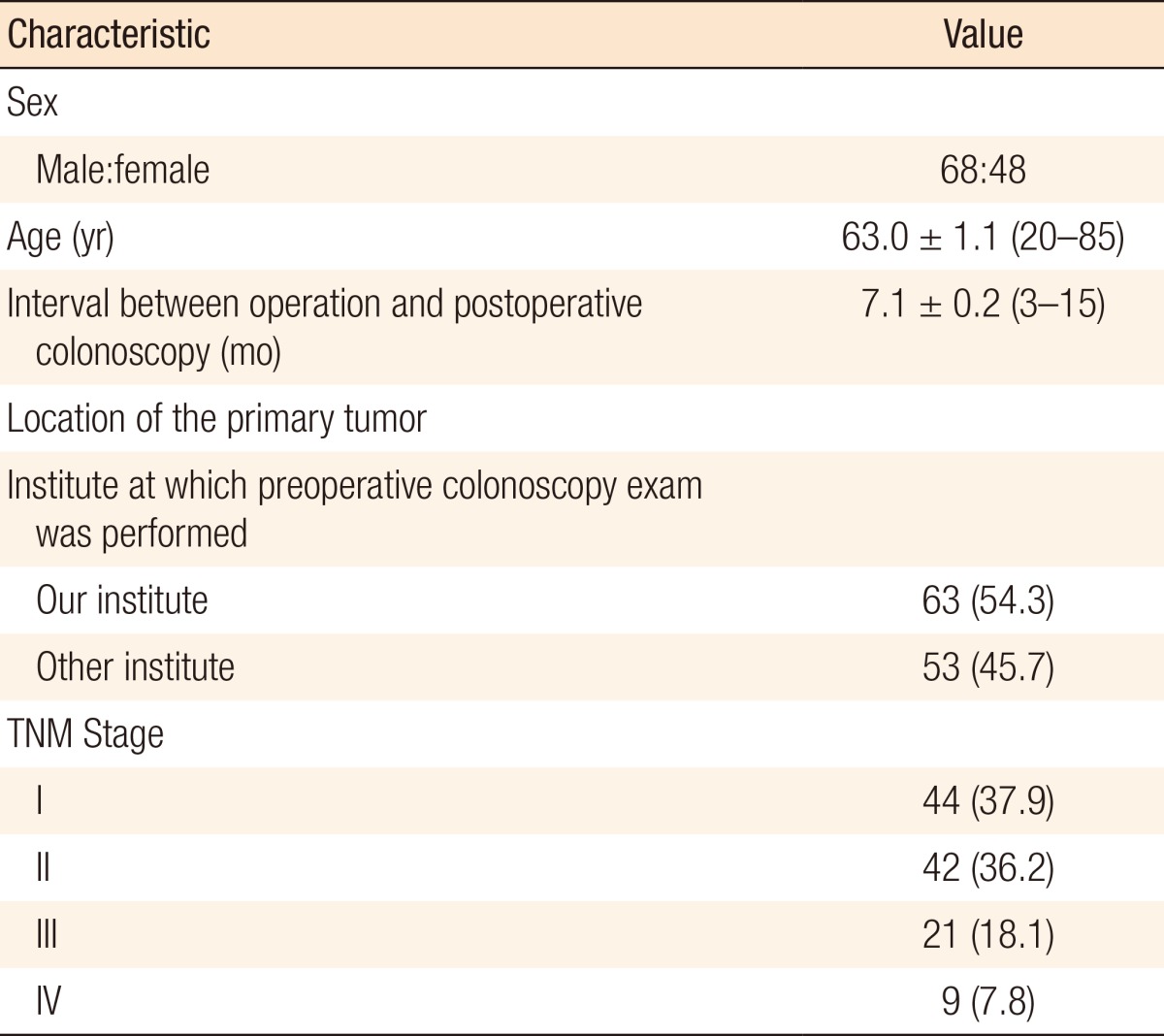
Among the 116 patients, 68 were males and 48 were females; their mean age was 63 years (range, 20-85 years). Postoperative colonoscopy was performed 7.1 months (mean) after radical surgery (range, 3-15 months). The location of the primary colorectal cancer was within the colon in 102 patients and within the rectum in 14 patients. Preoperative colonoscopy was performed in 63 patients at this institute and in 53 patients at other clinics. The TNM stage was stage I in 44 patients, stage II in 42 patients, stage III in 21 patients, and stage IV in 9 patients. The patients' characteristics are shown in Table 1. Polyps were classified according to their size (cm): <0.5; ≥0.5, <1.0; and ≥1.0.
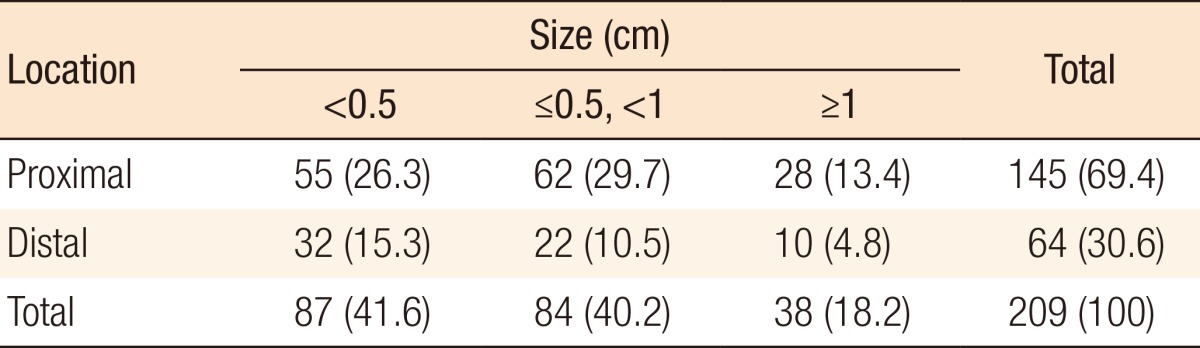
During preoperative colonoscopy, a total of 209 polyps were detected: for 87 polyps, size (cm) < 0.5; for 84 polyps, 0.5 ≤ size (cm) < 1.0; and for 38 polyps, size (cm) ≥ 1.0. Of the 87 polyps for which size (cm) < 0.5, 41 were located in the right colon, 35 were located in the left colon, and 11 were located in the rectum. Of the 84 polyps for which 0.5 ≤ size (cm) < 1.0, 40 were located in the right colon, 38 were located in the left colon, and 6 were located in the rectum. Of the 38 polyps for which size (cm) ≥ 1.0, 12 were located in the right colon, 22 were located in the left colon, and 4 were located in the rectum (Table 2). Table 3 shows the relationship between the polyps and the primary tumor during preoperative colonoscopy. One hundred 45 polyps (69.4%) from the primary tumor were located in the proximal colon (P < 0.001). The sizes of 38 of 209 polyps (18.2%) were ≥1.0 cm (P = 0.002).
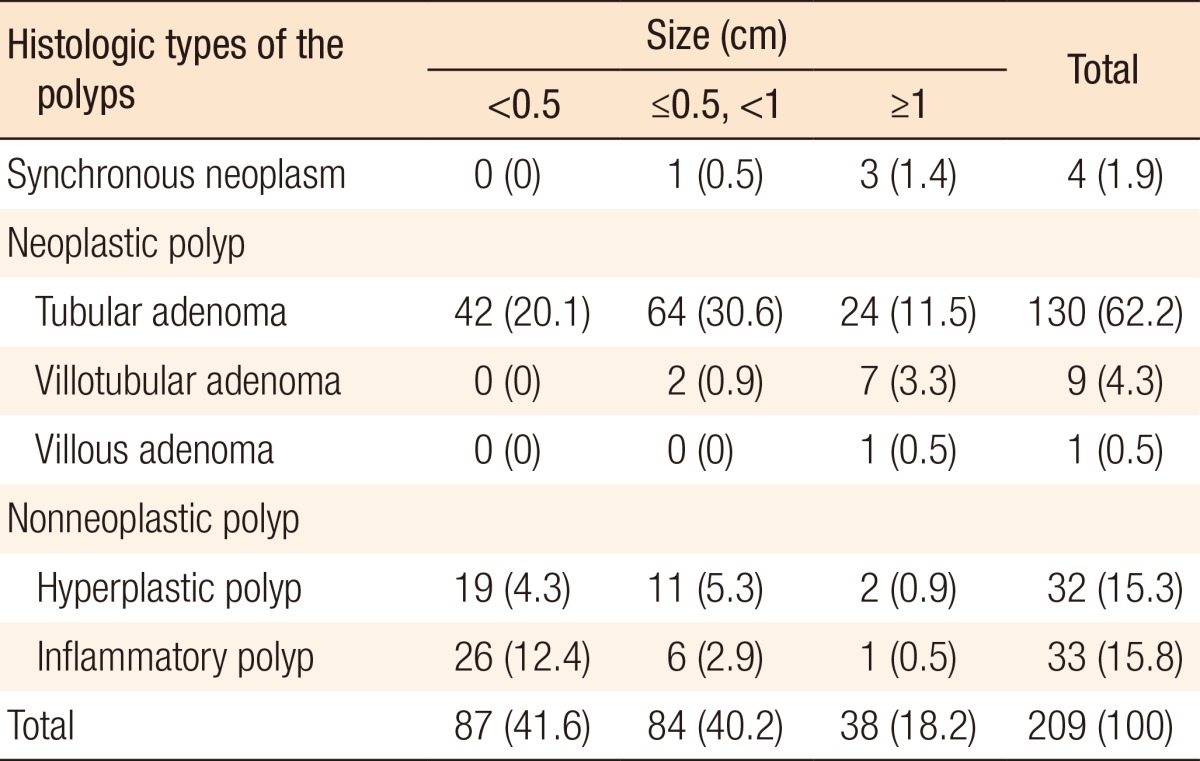
The histologic types of the polyps found during preoperative colonoscopy are presented in Table 4: 4 synchronous colorectal cancers (1.9%), 140 neoplastic polyps (67%), and 65 nonneoplastic polyps (31.1%). Among the 140 neoplastic polyps, the most common histologic types were tubular adenomas (130 polyps, 62.2%), villotubular adenomas (9 polyps, 4.3%), and a villous adenoma (1 polyp, 0.5%). Among the 65 nonneoplastic polyps, the incidences of hyperplastic polyps and inflammatory polyps were similar (15.3% and 15.8%, respectively).
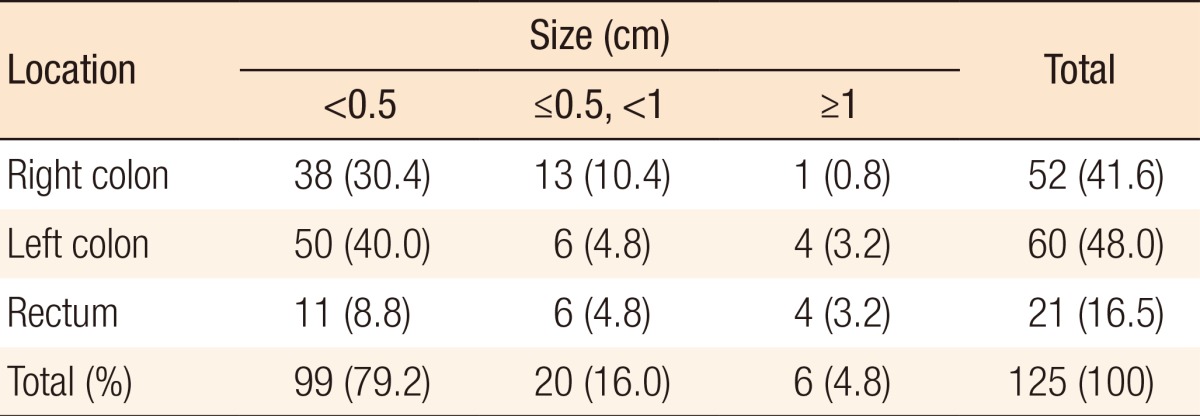
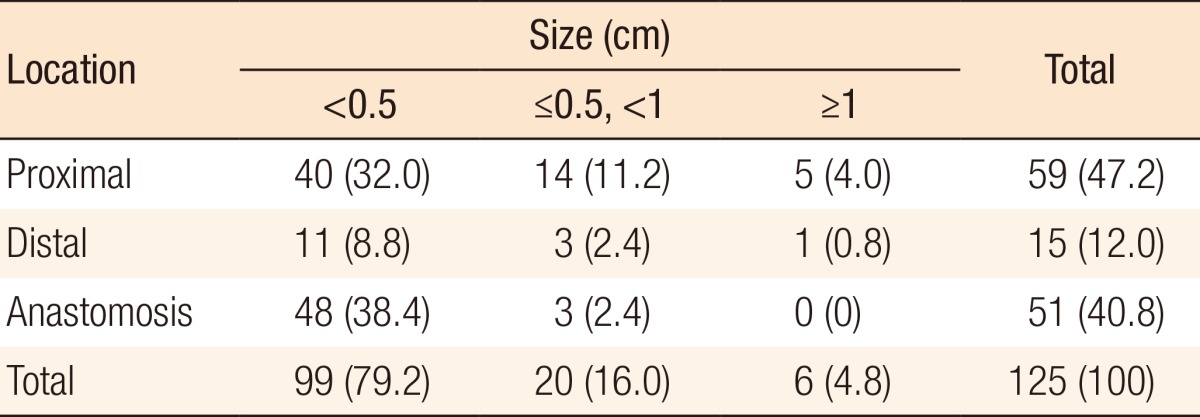
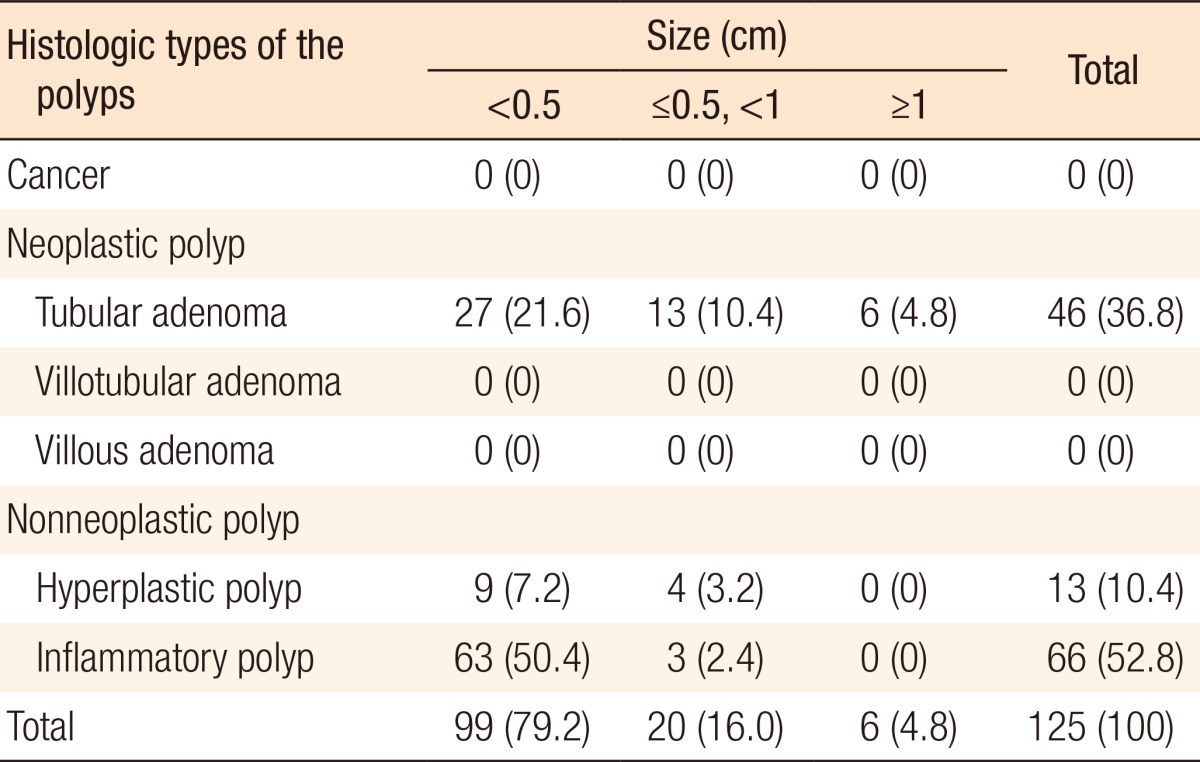
During postoperative colonoscopy, a total of 125 polyps were detected. The anatomical distributions of the polyps were similar between the preoperative and the postoperative colonoscopies. However, most of the polyps found during the postoperative colonoscopies had sizes < 0.5 cm (99 polyps, 79.2%) (Table 5). According to the location based on anastomosis, 59 polyps (47.2%) were located in the proximal part of the anastomosis, 15 (12%) were located in the distal part of the anastomosis, and 51 (40.8%) were located at the anastomotic site, as shown in Table 6 (P < 0.001). The histologic results for the polyps detected by postoperative colonoscopy are shown in Table 7. Among the 125 polyps, no cancerous polyps were detected; 46 (36.8%) were neoplastic polyps and 79 (63.2%) were nonneoplastic polyps. All neoplastic polyps (46 polyps) were tubular adenomas (Table 7). In the subgroup analysis of the neoplastic polyps detected by postoperative colonoscopy, neoplastic polyps were commonly located in the right colon and the proximal part of the anastomosis (60.9% and 82.6%, respectively, both P < 0.001) (Table 8).
In the 116 enrolled patients, the incidence of total polyps during postoperative colonoscopy was 53.4% (62 out of 116 patients), and the incidence of neoplastic polyps was 25.9% (30 out of 116 patients). According to the previous definition of the miss rate, the miss rate for the total number of polyps was 37.4%, and the miss rate for neoplastic polyps was 24.2%. A Spearman correlation analysis showed that the incidence of neoplastic polyps increased during postoperative colonoscopy just as the incidence of neoplastic polyps had increased during preoperative colonoscopy (r = 0.164, P = 0.048). On the other hand, no correlation was found between the detection of nonneoplastic polyps and that of neoplastic polyps (P = 0.252, postoperative; P = 0.410).
DISCUSSION
In this study, the miss rate for neoplastic polyps found during postoperative, but not preoperative, colonoscopy was 24.2%. Higher percentages of the missed neoplastic polyps were located in the right colon and the proximal part from the primary tumor percentage. The more neoplastic polyp detected during preoperative colonoscopy was associated with the more missed neoplastic polyp detected during postoperative colonoscopy.
In colorectal cancer patients, postoperative colonoscopic surveillance is the most useful strategy for early detection of polyps and metachronous colorectal cancer [7]. The NCCN guidelines recommend postoperative colonoscopy surveillance within the first year after a colorectal cancer resection [8]. If a patient is unable to undergo a complete colonoscopy preoperatively due to an obstructive lesion, colonoscopy should be performed within 3-6 months of the surgery. However, whether or not a patient who did not have a preoperative obstruction needs to undergo postoperative colonoscopy within the first year is a subject of debate [91011].
In most studies, postoperative colonoscopy was performed within the first year [412]. Bensen et al. [4] showed that the miss rate for all polyps, including neoplastic and nonneoplastic polyps, was 17% and that the miss rate for neoplastic polyps was 12%. Pickhardt et al. [13] estimated that the miss rate for neoplastic polyps was 12%. Patel and Ahnen reported that the miss rate for neoplastic polyps ranged from 17%-47% [1415]. Rex et al. [16] demonstrated that the pooled miss rates for nonneoplastic polyps and neoplastic polyps were 27% and 22%, respectively. In this aspect, the suggestion that colonoscopic surveillance should be performed within the first year seems persuasive. On the other hand, Cone et al. [17] proposed that extending the time to the first colonoscopy appears to be safe because the incidence of polyps with sizes ≥ 1 cm was 3.2%. At this institute, colonoscopic surveillance is performed 6-8 months after surgery. Despite the interval being shorter than those reported in other papers, the incidence of missed polyps was high, the miss rate for total polyps being 37.4% and the miss rate for neoplastic polyps being 24.2%. Especially, in our study, the sizes of most neoplastic polyps were <5 mm, and the incidence of polyps with sizes ≥ 1 cm was 4.8%. Therefore, whether one year is the proper time interval for postoperative colonoscopic surveillance is questionable.
Our study showed that the missed neoplastic polyps were commonly located in the right colon and the proximal part from the primary tumor. The reason for these results is not clear. However, recent studies proposed a hypothesis that the mechanisms of polyp formation differ depending on the location of the polyp. Several studies have suggested that proximal colon cancers were more often associated with microsatellite instability (MSI) - high, BRAF mutation. Also, they showed that distal colon cancers were related to chromosomal instability, epidermal growth factor receptor or human epidermal growth factor receptor 2 amplification, and overexpression of epiregulin [1718192021]. Considering that a neoplastic polyp might be a premalignant lesion for colorectal cancer, these different mechanisms for colorectal cancer between the right and the left colon might have led to the difference in the distributions of neoplastic polyps.
Kawai et al. [22] showed that the presence of synchronous polyps influenced the adenoma-free survival in patients who underwent a curative resection. Similarly, our study showed that a higher number of neoplastic polyps detected during preoperative colonoscopy was associated with a higher number of missed neoplastic polyps being detected during postoperative colonoscopy.
In addition, some studies have demonstrated a correlation between the detection of neoplastic polyps and the detection of nonneoplastic polyps in patients who undergo screening colonoscopy within the first year following surgery [2223]. However, no correlation between the detection of non-neoplastic polyps and that of neoplastic polyps was observed in the present study.
Although many studies have evaluated polyps in colorectal cancer patients, very few references have reported evaluations of the histologic results for the anastomotic site. Our study showed that the histologic types of the polyps at the anastomosis were nonneoplastic polyps, especially inflammatory polyps. These data were similar to those reported by Weinstock and Shatz [24] and suggest that the most commonly observed abnormality at the anastomosis is inflammatory polyps.
The probable reasons polyps were missed on the preoperative colonoscopy were incomplete bowel preparation, inappropriate withdrawal technique, inadequate polypectomy, and lower skill level of the endoscopist. Consequently, we should carefully evaluate the patient's history, such as family history, previous numbers of polyps, and histologic dysplasia in previous polyps. In addition, we should educate surgeons about good bowel preparation, determine the colonoscopic withdrawal time, and cooperate with the pathologist for achieving complete pathologic assessment.
In conclusion, polyps are located in the right colon during postoperative colonoscopy, especially in the proximal part from the primary tumor. Therefore, the endoscopist should carefully evaluate the right colon and the proximal part of the anastomosis during postoperative colonoscopic surveillance. In particular, patients in whom multiple neoplastic polyps were detected during preoperative colonoscopy should be closely and carefully followed.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.