Influence of Shorter Duration of Prophylactic Antibiotic Use on the Incidence of Surgical Site Infection Following Colorectal Cancer Surgery
Article information
Abstract
Purpose
This study aimed to identify the risk factors for surgical site infections (SSIs) in patients undergoing colorectal cancer surgery and to determine whether significantly different SSI rates existed between the short prophylactic antibiotic use group (within 24 hours) and the long prophylactic antibiotic use group (beyond 24 hours).
Methods
The medical records of 327 patients who underwent colorectal resection due to colorectal cancer from January 2010 to May 2014 at a single center were retrospectively reviewed, and their characteristics as well as the surgical factors known to be risk factors for SSIs, were identified.
Results
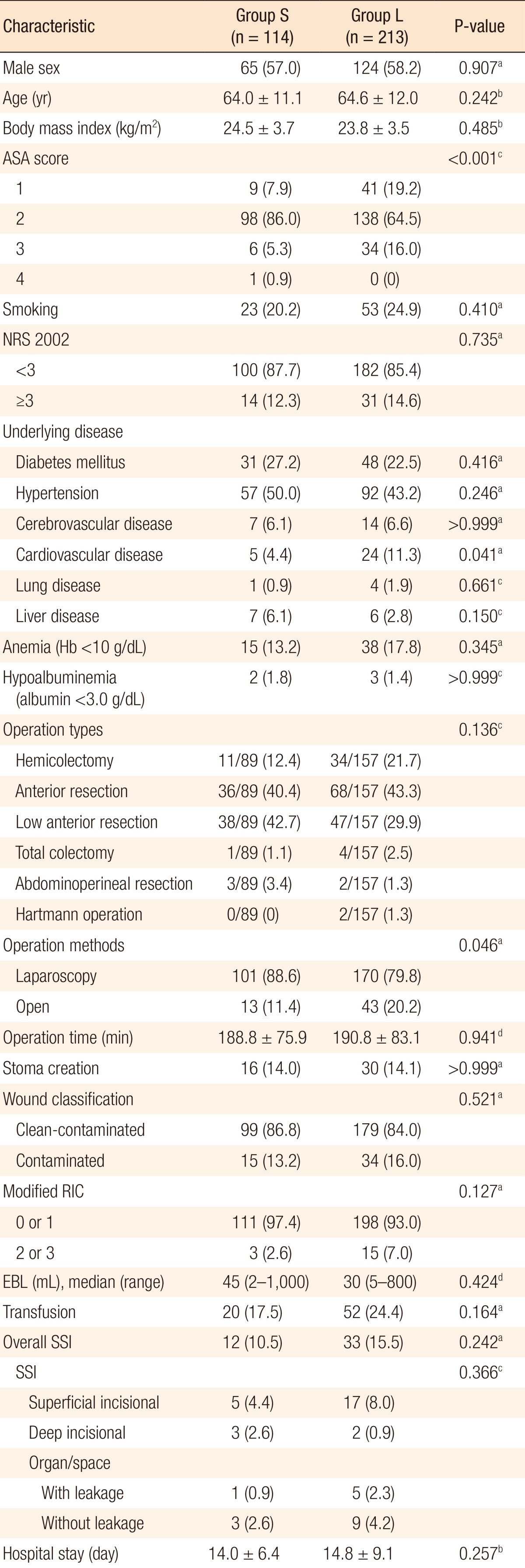
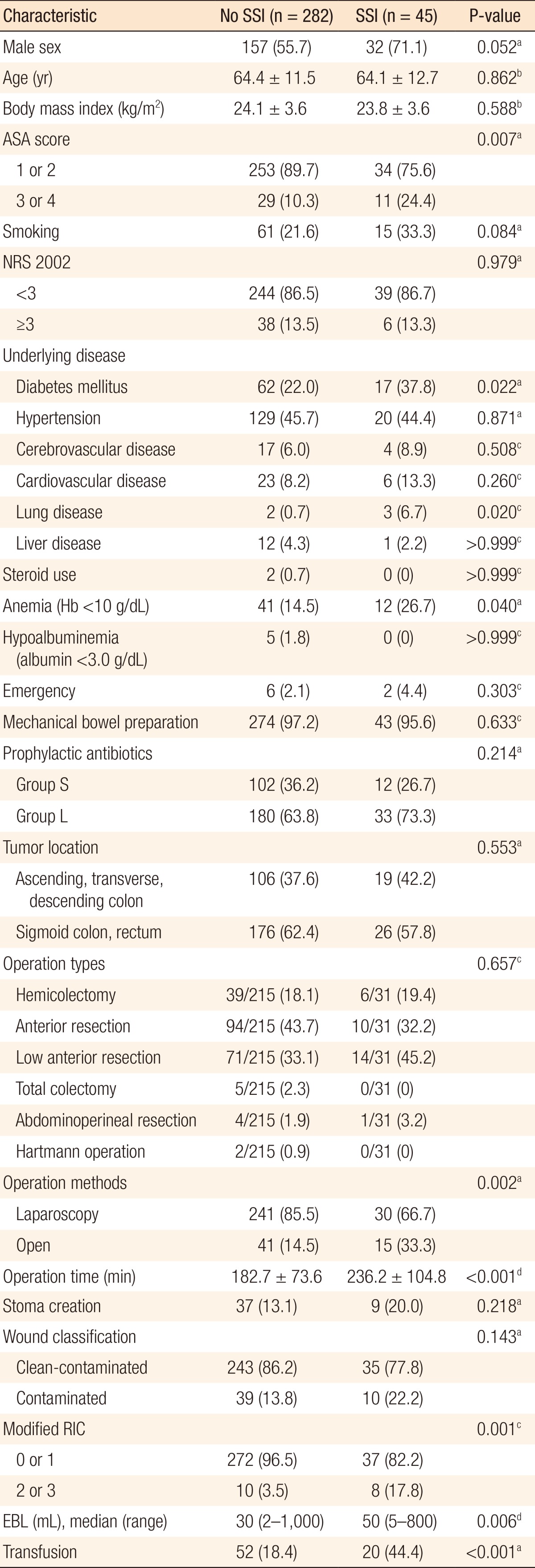
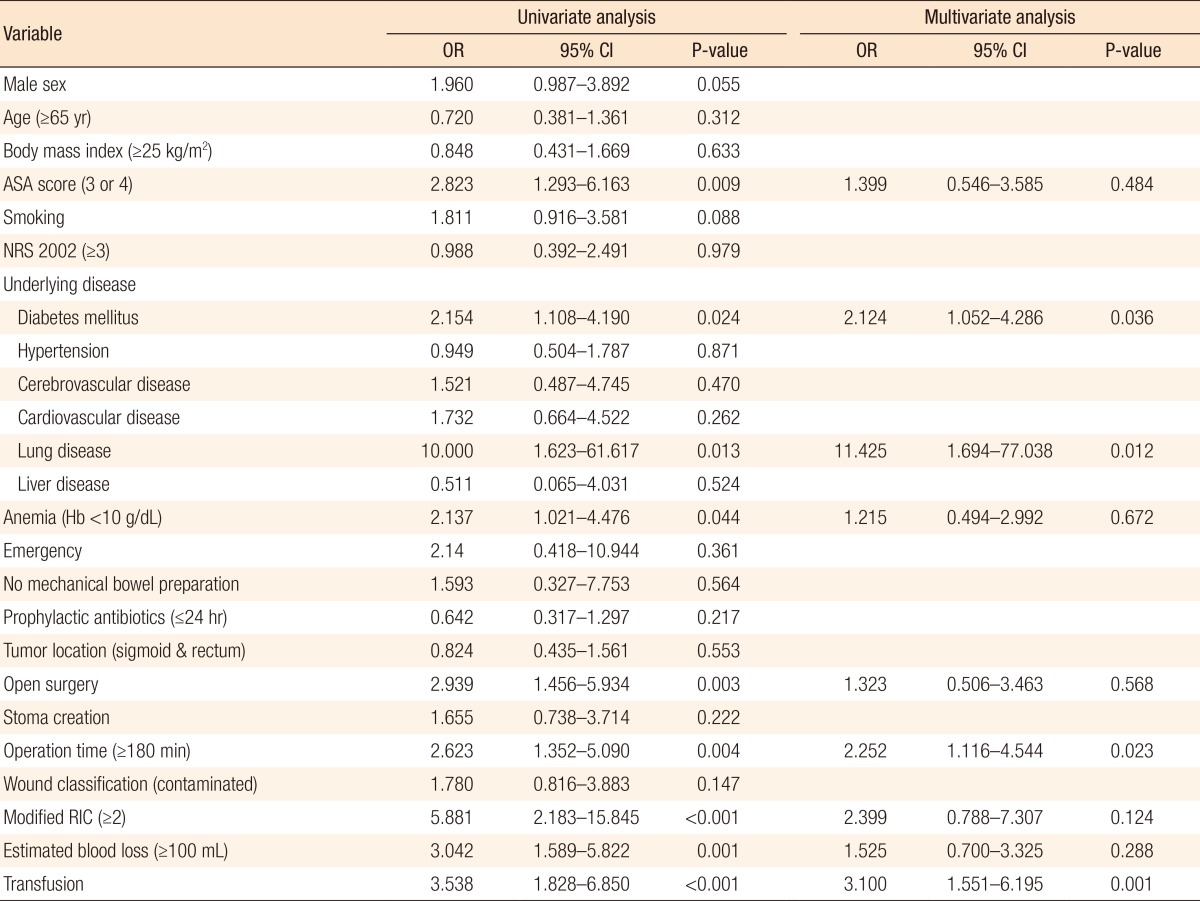
Among the 327 patients, 45 patients (13.8%) developed SSIs. The patients were divided into two groups according to the duration of antibiotic use: group S (within 24 hours) and group L (beyond 24 hours). Of the 327 patients, 114 (34.9%) were in group S, and 213 (65.1%) were in group L. Twelve patients (10.5%) in group S developed SSIs while 33 patients (15.5%) in group L developed SSIs (P = 0.242). History of diabetes mellitus and lung disease, long operation time, and perioperative transfusion were independent risk factors for SSIs.
Conclusion
This study shows that discontinuation of prophylactic antibiotics within 24 hours after colorectal surgery has no significant influence on the incidence of SSIs. This study also showed that history of diabetes mellitus and lung disease, long operation time, and perioperative transfusion were associated with increased SSI rates.
INTRODUCTION
Surgical site infection (SSI) is one of the common types of nosocomial infections along with urinary tract infection, pneumonia, and catheter-related bloodstream infection [1]. It is also a leading cause of the increases in the length of hospital stay and the cost [12]. SSI rates after colorectal surgery have been observed to range from 30% to 60% without prophylactic antibiotics, but prophylactic antibiotic use has significantly reduced the risk of SSIs by at least 75% [3456].
Prophylactic antibiotic administration has been found to be essential to reduce the morbidity and the mortality caused by SSIs. Meanwhile, the duration of prophylactic antibiotic use for colorectal surgery varies according to the policy of the institution and/or the experience of the surgeon [7]. Previous studies recommended an adequate duration of prophylactic antibiotic use after colorectal surgery in consideration of cost and the need to prevent the evolution of antimicrobial-resistant bacteria [489]. In addition, identifying the factors that influence SSI rates is important. Therefore, we performed a retrospective study to assess the adequate duration of antibiotic use and to identify risk factors for SSIs in patients who underwent colorectal cancer surgery.
METHODS
Data on the patients who underwent colorectal cancer surgeries at the Department of Colorectal Surgery, Kyung Hee University Hospital at Gangdong, from January 2010 through May 2014 were retrospectively reviewed and analyzed. Patients whose antibiotic administration was empirical or therapeutic for their septic condition due to cancer perforation or obstruction or for any other infection sources were excluded. The patients who were administrated antibiotics with prophylactic intent were included; a total of 327 patients were in the study population. In this population, majority of them underwent elective surgery except 8 patients who underwent emergency surgery due to cancer obstruction. The patients' characteristics and the perioperative variables known to have an association with SSIs were analyzed.
The patient characteristics were as follows: sex, age, body mass index (BMI), the American Society of Anesthesiologists (ASA) score, smoking, preoperative nutritional status, underlying diseases, and hemoglobin levels. The smoking group included patients who had smoked until the time of their admission, and the no-smoking group included nonsmokers who had never smoked and ex-smokers who had quit smoking at least 1 month before their admission. Anemia was defined as a serum hemoglobin level less than 10 g/dL, and hypoalbuminemia was defined as a serum albumin level less than 3 mg/dL. The reference levels were taken from a previous study on SSIs [10]. The nutritional status of the patients was measured by using Nutritional Risk Screening (NRS) 2002 [11]. NRS 2002 is a nutritional screening tool used to identify patients who need nutritional support; it was established by members of an ad hoc working group under the auspices of the European Society for Clinical Nutrition and Metabolism Educational Committee. Patients with a score of three or more were considered nutritionally at-risk.
The perioperative variables were as follows: emergency, mechanical bowel preparation (MBP), duration of prophylactic antibiotic use, tumor location, operation type, operation method, stoma creation, operation time, wound classification, modified risk index category (RIC), estimated blood loss (EBL), and perioperative blood transfusion. The modified RIC, which was proposed by the Centers for Disease Control and Prevention (CDC)'s National Nosocomial Infections Surveillance (NNIS) system, was adapted to investigate the association with SSI rate [12]. In the 1970s, the RIC was first developed based on three variables, which were the ASA score, operation time, and wound classification according to the CDC's NNIS system. It was modified in 2001 because of the popularization of laparoscopic surgeries. Points are added for operation times over 180 minutes, ASA score of three or more, or wound classification of three or more (i.e., contaminated or dirty wounds), but a point is subtracted when a laparoscopic surgery is performed. According to the NNIS system, SSIs were categorized as superficial, deep, or organ/space SSIs which occurred within 30 days after the surgeries [13]. To evaluate the influence of the duration of prophylactic antibiotic use on the SSI rates, we divided the patients into two groups according to the duration of prophylactic antibiotic use: group S (within 24 hours) and group L (beyond 24 hours).
Preoperative MBPs were performed with polyethylene glycol (4 L) in patients without cancer obstruction. All patients received second generation cephalosporins, including cefotetan, cefminox, and cefotiam, at a dosage of 1 g twice a day intravenously. We assumed that the administration of the antibiotic was discontinued within 12 hours following the time of administration of the last dose indicated in the patient's medical record. The preoperative dose was administered after induction of general anesthesia, prior to skin incision. No intraoperative boost doses were administered.
Statistical analyses were performed by using PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA). The chi-square test or Fisher exact test was used for the categorical variables, and the Student t-test was used for the continuous variables. Subsequently, a multivariate analysis was performed by using a logistic regression. The variables with a P-value of less than 0.05 in the univariate analysis were included in the multivariate analysis.
RESULTS
After the patients who had undergone emergent surgery due to cancer perforation or who had already been administered antibiotics for any reasons had been excluded, a total of 327 patients were included in this study. Their mean age was 64.4 ± 11.7 years, and the number of male patients was 189 (57.8%). Most patients had an ASA score of one or two (286, 87.5%), and 53 patients (16.2%) had anemia. With respect to perioperative outcomes, the wounds of 278 patients (85%) were classified as clean-contaminated wounds, and the wounds of 49 patients (15%) were classified as contaminated wounds. The mean operation time was 190.1 minutes. Forty-six patients (14.1%) underwent a stoma creation. A right hemicolectomy, transverse colectomy, and left hemicolectomy accounted for 28.5%, 2.1%, and 8.0% of the operations, respectively. Anterior and low anterior resections together accounted for 57.8% of the operations. A total colectomy, abdominoperineal resection, and Hartmann operation accounted for 3.6% of the operations. Fifty-six patients (17.1%) underwent open surgeries, and 72 patients (22%) received perioperative blood transfusion.
SSIs developed in 45 patients (13.8%). Twenty-two patients (6.7%) had superficial SSIs, five patients (1.5%) had deep SSIs, and 18 patients (5.5%) had organ/space SSIs. One hundred fourteen patients were included in group S, and the average duration of the prophylactic antibiotic administration was 23.8 ± 0.2 hours. Two hundred thirteen patients were included in group L, and the average duration of prophylactic antibiotic administration was 54.7 ± 19.9 hours. On comparing groups S and L, most of the patients' demographics were not different except for the ASA score, the incidence of cardiovascular disease, and the rate of open surgery (Table 1). Postoperative outcomes, such as the SSI rates and the hospital stay, were also not statistically different.
ASA score, underlying diabetes mellitus (DM) and lung disease, anemia, open surgery, long operation time, high RIC, large amount of EBL, and perioperative transfusion were related with SSIs in the univariate analysis (Table 2). Variables were dichotomized as follows: age <65 years vs. age ≥65 years (median age of the patients), ASA score of 1 and 2 vs. ASA score of 3 and 4, NRS 2002 score <3 vs. NRS 2002 score ≥3, prophylactic antibiotic use within 24 hours vs. prophylactic antibiotic use beyond 24 hours, tumor located in the ascending, transverse, and descending colon vs. tumor located in the sigmoid colon and rectum, operation time <180 minutes vs. operation time ≥180 minutes (same as the operation time determinant of RIC), modified RIC of 0 and 1 vs. modified RIC of 2 and 3, EBL of <100 mL vs. EBL of ≥100 mL (median EBL in the patients). Among the variables that showed an association with the SSI rates in the univariate analysis, underlying diseases including DM and lung disease, the operation time, and perioperative transfusion were proven to be associated with the SSI rates in the multivariate analysis (Table 3).
DISCUSSION
SSI rates after colorectal surgery have been reported to range from 3% to 43% [810121415]. In the United States, the National Healthcare Safety Network reported in 2009 that SSIs occurred in 4%–9% of the patients who underwent colon surgery and in 3%–27% of the patients who underwent rectal surgery [16]. In Japan, the Japan Nosocomial Infection Surveillance reported in 2014 that the SSI rates after colon and rectal surgeries were 15.0% and 17.8%, respectively, based on data collected from 2008 to 2010 [17]. In South Korea, the Korean Surgical Site Infection Surveillance (KOSSIS) reported in 2014 that the SSI rates after a colectomy and a proctectomy were 10.15% and 13.54%, respectively. In comparison to the previous reports about the SSIs after colorectal surgeries, the SSI rate of 13.7% (13.0% after a colectomy and 15.5% after a proctectomy) in this study can be considered reasonable.
SSI rates without prophylactic antibiotic use have been reported to range from 30% to 60%. In an analysis of clinical trials of prophylactic antibiotic use for colon procedures, a significantly lower mortality rate of 4.5% was noted in the prophylactic antibiotic use group compared to the mortality rate of 11.2% in the control group [345]. Due to the somewhat large burden of high mortality and morbidity caused by SSIs after colorectal surgery, many surgeons tend to extend the duration of prophylactic antibiotic use, despite the guidelines recommending discontinuation of prophylactic antibiotics within 24 hours after colorectal surgery [418]. In the United States, a national retrospective cohort study conducted in 2001 revealed that prophylactic antibiotics were discontinued within 24 hours of the surgery end time in only 40.8% of the patients who underwent colon surgery, and the median time to discontinuation of prophylactic antibiotics was 57 hours [19]. Moreover, in a 2013 survey of Korean colorectal surgeons with respect to prophylactic antibiotic use, only 12.3% of the surgeons stopped prophylactic antibiotics within 24 hours after surgery, 31.5% of the surgeons used prophylactic antibiotics until postoperative day 3, and 13.7% of the surgeons used prophylactic antibiotics beyond postoperative day 5 [7].
However, in the light of the need to reduce cost and to minimize the evolution of antibiotic-resistant bacteria, prophylactic antibiotics should be discontinued early as long as no evidence exists that prolonged prophylactic antibiotic use guarantees a much lower SSI rate. Many researchers have advocated short-term use of prophylactic antibiotics, Cochrane Review is a journal as was reported in a meta-analysis in Cochrane Review in 2014 [20]. Nevertheless, as mentioned above, the surveys on actual trends of prophylactic antibiotic use among colorectal surgeons have revealed that the majority used prophylactic antibiotics within 24 hours. In addition, the optimal duration for prophylactic antibiotic use after colorectal surgery is a controversial issue. From that point of view, some studies have recently compared the SSI rates for various durations of usage of prophylactic antibiotics [8921]. In 2010, a prospective multicenter randomized trial comparing SSI rates between the three-day prophylactic antibiotic use group and the five-day prophylactic antibiotic use group for elective colorectal surgeries was conducted in South Korea, and no difference in the overall SSI rates (3.1% vs. 2.4%) was noted [8]. In Japan in 2007, a prospective randomized trial comparing incisional SSI rates between the single-dose prophylactic antibiotic use group and the three-dose prophylactic antibiotic use group showed a lower SSI rate in the three-dose prophylactic antibiotic use group (14.2% in the single-dose group vs. 4.3% in the three-dose group) [21]. Also, a prospective study about 24-hour use of prophylactic antibiotics showed a SSI rate of about 10%. However, the number of patients enrolled in that study was only 69, and it was a single-arm study [9]. Based on those studies, we decided to compare the 24-hour prophylactic antibiotic use group and the over 24-hour prophylactic antibiotic use group for patients who had undergone colorectal cancer surgery.
In this study, discontinuation of prophylactic antibiotics within 24 hours after colorectal surgery did not increase the SSI rate. Groups L and S had SSI rates of 15.5% and 10.5%, respectively. The SSI rate in group L was higher than that in group S, but the difference was not statistically significant. However, statistically, the ASA score in group L was higher than that in group S (P < 0.001), and more patients in group L had cardiovascular disease (P = 0.041) and underwent open surgery (P = 0.046) compared to the patients in group S. In this study, prolonged antibiotic use might have been applied due to conditions such as underlying disease or open surgery, which resulted in a longer incision.
Along with the comparison of the SSI rates between group S and group L, risk factors for SSIs were analyzed. In the univariate analysis, higher ASA score, underlying DM, lung disease, anemia, open surgery, longer operation time, more EBL, perioperative transfusion, and modified RIC of 2 or 3 were statistically related to SSIs. Among these variables with statistical significance, DM, lung disease, operation time, and transfusion were independent risk factors for SSIs. Higher ASA score means that a patient has a more severe systemic disease, but it does not indicate the type of disease entity. Therefore, the fact that high ASA score was not found to be an independent risk factor in the subsequent multivariate analysis implicates that specific diseases are more important for SSIs. In this study, the specific systemic diseases related to SSIs were DM and lung disease. These two conditions lead to impaired tissue oxygenation and cause reduced collagen synthesis and impaired neutrophil function [222324]. Moreover, the hyperglycemic status of DM patients also accelerates nonenzymatic glycosylation of proteins, thereby inactivating immunoglobulin and hindering opsonization of bacteria [2526]. In addition, newly synthesized collagen can be easily glycosylated; therefore, wound collagen is reduced via increased collagenase activity. Therefore, a helpful clinical implication would be whether or not perioperative glycemic control affects the SSI rates in nondiabetic patients. Kiran et al. [27] reported that even a single event of postoperative hyperglycemia (defined as >125 mg/dL) was associated with an increased morbidity and mortality rate after colorectal surgery; hence, they suggested monitoring the serum glucose level and tight glucose control even in nondiabetic patients. However, glycosylated hemoglobin levels and perioperative blood glucose levels were not available in all patients in our study because of the retrospective nature of the data collection.
Tissues manipulated during operation inevitably received reduced tissue oxygenation to some degree due to disruption of the vascular supply or thrombosis of the vessels [28]. Factors causing reduced tissue oxygenation can affect the SSIs because the primary defense mechanism of a host is oxidative killing by neutrophils, and infection risk is associated with tissue's oxygen partial pressure [22]. In this study, we expected that a longer operation time and the use of an open method would increase the SSI rates because of a possibly broader area of tissue manipulation and a higher probability of a encountering a technically difficult situation. As expected, in the univariate analysis, open surgery and longer operation time were associated with increased SSI rates, but in multivariate analysis, open surgery was not an independent risk factor for SSIs. In a comparison of the operation times, the mean operation time for open surgeries was longer than that for laparoscopic surgeries (210.5 ± 97.1 minutes vs. 185.9 ± 76.2 minutes, P = 0.079); hence, the operation time could confound the result.
If an operation takes too long or the amount of blood loss is high, we can expect the tissue concentration of prophylactic antibiotics to be lower than the minimum inhibitory concentration and the tissue's oxygenation to be decreased. With respect to anemia, one might instinctively think that it would be an inevitable cause of tissue hypoxia. However, preoperative anemia results in an increase of cardiac output and oxygen extraction to maintain tissue oxygenation as long as the intravascular volume is maintained. Therefore, anemia itself does not cause impaired tissue oxygenation in normovolemic patients [29]. We were able to confirm this fact in the multivariate analysis. In addition, in this study, the patients with anemia tended to be transfused perioperatively (64.2%); hence, the adverse effect of transfusion on SSIs could have acted as a confounding factor. Transfusion-related immunosuppression could be the main cause of the increased SSI rate [30]. Similar to anemia, EBL could be confounded by transfusion and long operation time. This is because the proportion of the transfused patients in the patients with EBL of ≥ 100 mL was larger than that in the patients with EBL of < 100 mL (44.0% vs 14.0%, P < 0.001), and the mean operation time was longer in the patients with EBL ≥ 100 mL than that in the patients with EBL > 100 mL (243.7 ± 95.77 minutes vs 171.6 ± 65.20 minutes, P < 0.001). Lastly, the modified RIC, which is a known predictive index of SSIs, was not an independent risk factor for SSIs in the multivariate analysis. This could be due to the facts that the ASA scores and the wound classifications among the patients did not vary; furthermore, the operation method was not an independent risk factor for SSIs in this study. The main factor that could affect the SSI rate from among the determinants of the modified RIC was only the operation time (184.4 ± 77.2 minutes vs. 288.6 ± 73.7 minutes, P < 0.001).
A few limitations of this study should be considered. First, this study was retrospective in nature; hence, some biases might be present. More patients in group L had a high ASA score than patients in group S; hence, the question about the effect of prolonged duration of antibiotic prophylaxis in patients with a high ASA score persists. Despite the pitfalls, this study can provide clinical implications for SSIs.
In conclusion, discontinuation of prophylactic antibiotics within 24 hours after colorectal surgery does not increase the SSI rate compared to prolonged prophylactic antibiotic use. In light of the need to reduce cost and prevent the evolution of bacteria with antimicrobial resistance, prophylactic antibiotic use within 24 hours after surgery should be recommended. In addition, for the prevention of SSIs, attention should be given to the management of patients with underlying DM or lung disease and to the duration of surgery and the amount of perioperative transfusion.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.