Multivariate Analysis of Risk Factors Associated With the Nonreversal Ileostomy Following Sphincter-Preserving Surgery for Rectal Cancer
Article information
Abstract
Purpose
A loop ileostomy is used to protect an anastomosis after anal sphincter-preserving surgery, especially in patients with low rectal cancer, but little information is available concerning risk factors associated with a nonreversal ileostomy. The purpose of this study was to identify risk factors of ileostomy nonreversibility after a sphincter-saving resection for rectal cancer.
Methods
Six hundred seventy-nine (679) patients with rectal cancer who underwent sphincter-preserving surgery between January 2004 and December 2011 were evaluated retrospectively. Of the 679, 135 (19.9%) underwent a defunctioning loop ileostomy of temporary intent, and these patients were divided into two groups, that is, a reversal group (RG, 112 patients) and a nonreversal group (NRG, 23 patients) according to the reversibility of the ileostomy.
Results
In 23 of the 135 rectal cancer patients (17.0%) that underwent a diverting ileostomy, stoma reversal was not possible for the following reasons; stage IV rectal cancer (11, 47.8%), poor tone of the anal sphincter (4, 17.4%), local recurrence (2, 8.7%), anastomotic leakage (1, 4.3%), radiation proctitis (1, 4.3%), and patient refusal (4, 17.4%). The independent risk factors of the nonreversal group were anastomotic leakage or fistula, stage IV cancer, local recurrence, and comorbidity.
Conclusion
Postoperative complications such as anastomotic leakage or fistula, advanced primary disease (stage IV), local recurrence and comorbidity were identified as risk factors of a nonreversal ileostomy. These factors should be considered when drafting prudential guidelines for ileostomy closure.
INTRODUCTION
The treatment of rectal cancer, especially low to mid rectal cancer, has moved toward sphincter preservation during the past decades because surgeons and patients prefer this option to an abdominoperineal resection (APR) [1]. Furthermore, due to the wide adoption of preoperative chemoradiation therapy and a total mesorectal excision, the technique of primary anastomosis after resection with a temporary diverting stoma has evolved over several decades, and as a result, the sphincter-preservation rate continues to increase in patients with rectal cancer [234].
However, sphincter-saving surgery with a lower level of anastomosis increases the risk of anastomosis-related complications, such as leakage, fistula and stricture [156]. In fact, the prevalence of anastomotic leakage has been reported to vary from 1% to 39% after sphincter-preserving surgery [6]. Local recurrence and systemic metastasis are viewed as the main risk factors associated with stoma permanence [78910]. However, few studies [78] have sought to identify the risk factors associated with a nonreversal ileostomy; thus, in the present study, we attempted to identify the risk factors after a sphincter-saving resection for rectal cancer.
METHODS
Six hundred seventy-nine rectal cancer patients that underwent sphincter-preserving surgery at Gachon University Gil Medical Center between January 2004 and December 2011 were evaluated retrospectively. Of these, 523 were excluded because of no stoma formation, palliative surgery, hereditary nonpolyposis colorectal cancer, familial adenomatous polyposis, nonadenocarcinoma, and emergency operation. Of the 679, 135 underwent a defunctioning loop ileostomy of temporary intent to protect the distal anastomosis following an anterior resection (AR), a low anterior resection (LAR) and an ultralow anterior resection (uLAR). A standard tumor-specific mesorectal excision with autonomic nerve preservation was performed routinely. Lateral pelvic lymph node dissection was not routinely performed.
Medical records were reviewed retrospectively, and the data obtained were analyzed according to the intention-to-treatment principle. All relevant data were reviewed by the authors. The following parameters were analyzed: age, gender, body mass index (BMI), American Society of Anesthesiologists (ASA) classification, comorbidities (e.g., hypertension, diabetes, pulmonary disease), history of previous abdominal surgery, TNM tumor stage (according to the American Joint Committee on Cancer), local recurrence, cancer location, perioperative concurrent chemoradiation therapy (CCRT), type of operation, and postoperative morbidity.
Anastomotic leakage or fistula was defined as the clinical signs of peritonitis with radiologic findings, such as an intestinal wall defect, an abscess with air content at the anastomotic site, and a discharge of pus or fecal material through the pelvic drain. Anal or anastomotic stricture was diagnosed when a colonofiberscope with a distal tip of 13.2 mm in outer diameter was not passed.
Patients were followed up every 3 months for the first 2 years after surgery and then every 6 months until 5 years after surgery. All underwent blood tests and physical examination, including a rectal examination, at each visit, and serum carcinoembryonic antigen, carbohydrate antigen 19-9, and abdominopelvic computed tomography and colonofiberscopy findings were regularly checked. The 135 that underwent a defunctioning loop ileostomy were divided into two groups: 112 patients in the reversal group (RG) and 23 patients in the nonreversal group (NRG). In this study, a nonreversal stoma was defined as a loop ileostomy without reversal within three years of surgery.
Categorized variables were analyzed using the chi-square test and the Fisher exact test. Continuous variables are expressed as means ± standard deviations and were analyzed using the Student t-test. Variables with a P-value <0.10 were subjected to a multivariate analysis, which was conducted by using a logistic regression. The statistical analyses were performed by using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA), and statistical significance was accepted for P-values <0.05.
RESULTS
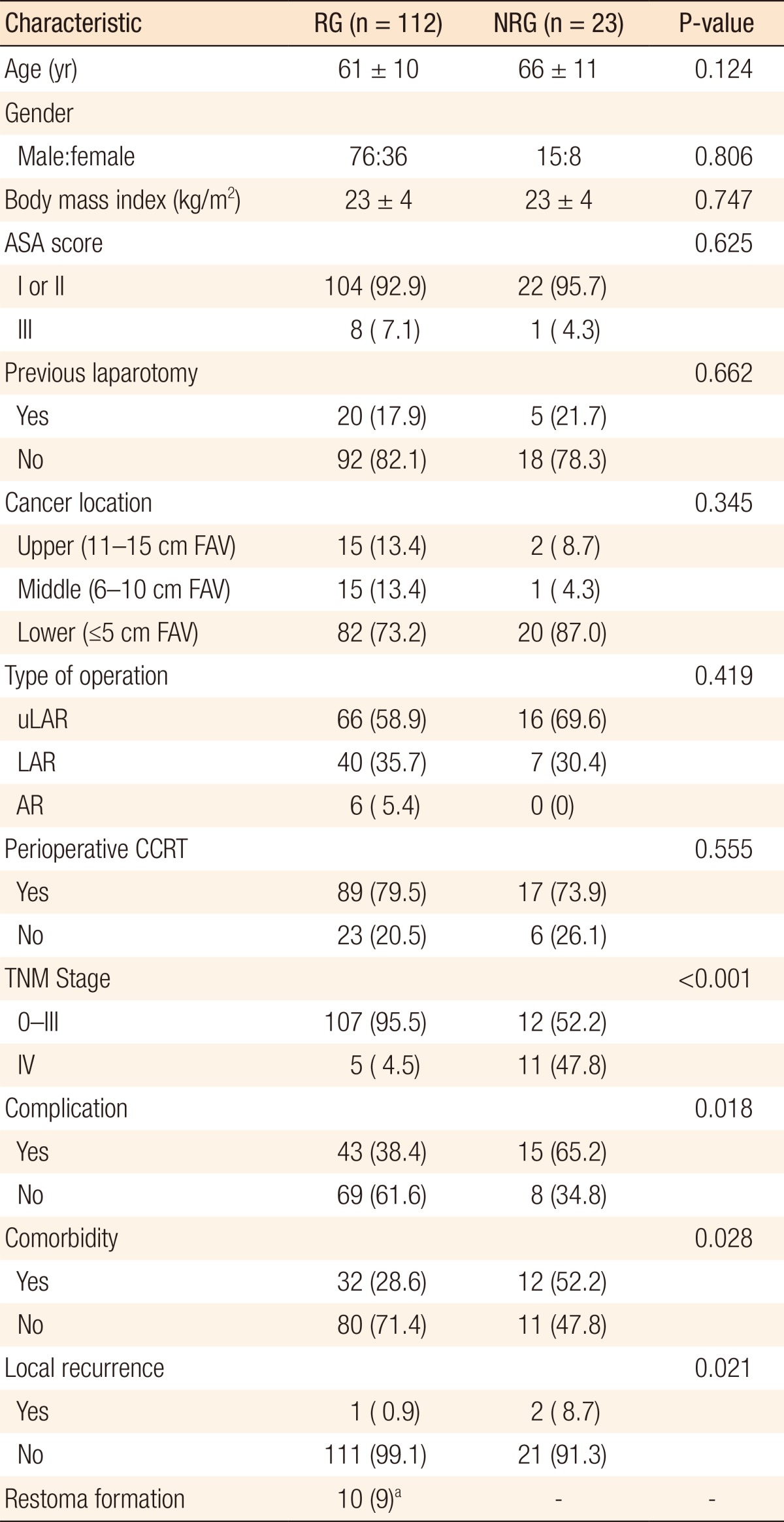
The mean follow-up was 48 months, and the mean time between main surgery and ileostomy takedown was 7.4 months. In order to protect the anastomosis, 135 of the 679 rectal cancer patients (19.9%) who received AR, LAR, or uLAR underwent a diverting ileostomy. In 23 of these 135 (17.0%), stoma reversal was not performed. The clinico-pathologic characteristics of patients in the RG and the NRG are detailed in Table 1. Of 135 study subjects, 91 were men and 44 were women, and in the NRG, 15 were men (16.5%) and 8 were women (18.2%). The mean ages in the RG and the NRG were 61 and 66 years, respectively, and the mean BMIs were identical at 23 kg/m2. Furthermore, the ASA grade, cancer location, type of operation, perioperative CCRT, and history of abdominal surgery were comparable in the two groups, but postoperative complications (P = 0.018), comorbidities (P = 0.028), advanced stage (P < 0.001), and local recurrence (P = 0.021) were significantly different.
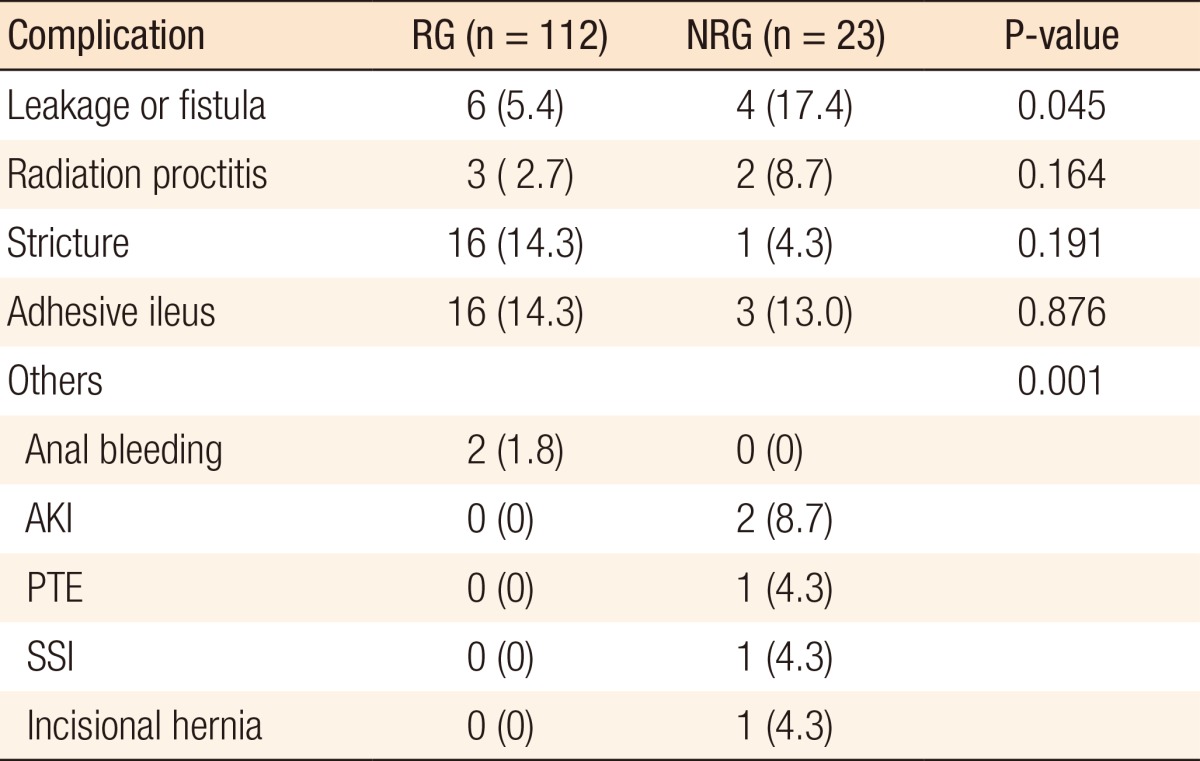
Postoperative complications, such as anastomotic leakage or fistula, radiation proctitis, anal or anastomotic stricture, adhesive ileus, and others, which included anal bleeding, acute kidney injury, pulmonary thromboembolism, surgical site infection, and incisional hernia, are detailed in Table 2. The most frequent complications in the RG were anastomotic stricture (16, 14.3%) and adhesive ileus (16, 14.3%), and that in the NRG was leakage or fistula (4, 17.4%). Of these, only anastomotic leakage or fistula (P = 0.045) was statistically significant.
In the RG (n = 112), 10 patients underwent a reoperation for stoma diversion (7 loop ileostomies, 2 transverse colostomies, and 1 Hartmann operation). In three of the 10, the stoma was taken down. The reasons for failure to take down were a radiation-related recto-vaginal fistula in 5, a mechanical ileus in 3, ischemic colitis in one, and delayed anastomotic leakage in another.
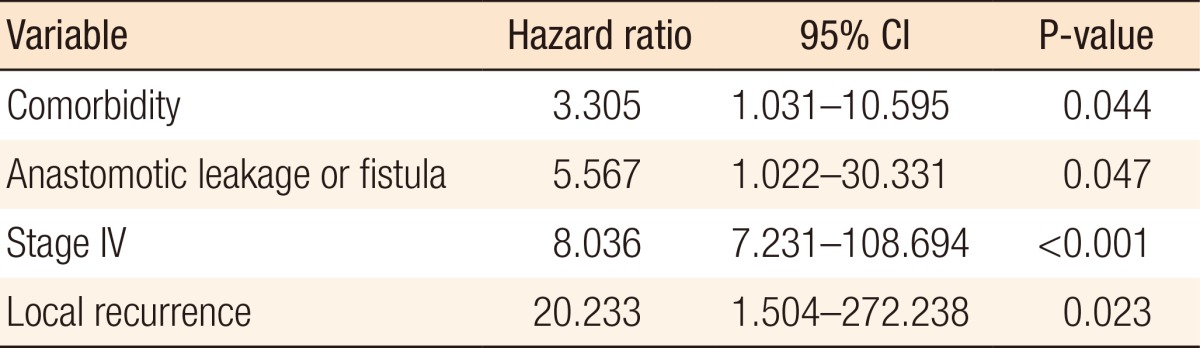
The results of the multivariate analysis are presented in Table 3. The independent risk factors for a nonreversal ileostomy were comorbidity (hazard ratio [HR], 3.305; 95% confidence interval [CI], 1.031-10.595, P = 0.044), anastomotic leakage or fistula (HR, 5.567; 95% CI, 1.022-30.331; P = 0.047), advanced cancer (stage IV) (HR, 28.036; 95% CI, 7.231-108.694; P < 0.001), and local recurrence (HR, 20.233; 95% CI, 1.504-272.238; P = 0.023). The causes of the 23 nonreversals were stage IV cancer (11, 47.8%), poor tone of the anal sphincter (4, 17.4%), local recurrence (2, 8.7%), anastomotic leakage (1, 4.3%), radiation proctitis (1, 4.3%) and patient refusal due to advanced age or poor economic status (4, 17.4%).
DISCUSSION
APR for rectal cancer requires a permanent stoma, but an AR of the rectum with a low level of anastomosis and a temporary defunctioning stoma enables anal sphincter preservation. Furthermore, sphincter-preserving surgery for rectal cancer does not increase the risk of recurrence as compared with APR [11] and improves quality of life by conserving normal bowel function. For these reasons, sphincter-preserving surgery is the treatment of choice for rectal cancer [12].
Some patients may undergo a temporary protective ileostomy accompanied by sphincter-preserving surgery because anastomotic complications are more likely for a low level of anastomosis and in patients with a history of neo-adjuvant CCRT for rectal cancer [813]. Although a protective ileostomy does not reduce the rate of anastomotic leakage, it can decrease the rate of symptomatic anastomotic leakage and re-operation [31314].
Some patients that undergo sphincter-preserving surgery with a protective defunctioning stoma may experience a permanent course as the nonreversal rate of a diverting loop ileostomy has been reported to range from 13.8% to 24.9% [10151617]. In the present study, 19.9% of the patients (135/679) who received sphincter-preserving surgery for rectal cancer received a protective loop ileostomy, and in 17% of the patients (23/135) that underwent radical surgery with a diverting ileostomy, the condition was nonreversible. Furthermore, 12% of the patients that underwent loop ileostomy closure re-experienced anastomotic leak [18]. In addition, we found that 10 of the 112 patients underwent stoma reconstruction because of radiation-related recto-vaginal fistula (5), mechanical ileus (3), ischemic colitis (1), or delayed anastomotic leakage (1). Delayed anastomotic leakage developed in only one patient (0.9%) in the RG. Most patients that underwent stoma reconstruction were affected by complications associated with radiation therapy, such as recto-vaginal fistula. Stoma repair was possible in only 3 of the 10 patients.
Stage IV disease, anastomosis-related complications, local recurrence, perioperative radiation therapy, an advanced age (over 70 years), male gender and uLAR have been suggested to be risk factors of stoma non-reversibility [891015192021]. Some authors have suggested that advanced malignant disease can impact the patient's general condition, prolong adjuvant chemotherapy, and induce a nonreversal stoma [15]. Lemmens et al. [22] reported that older colorectal cancer patients with comorbidity are less aggressively treated than patients without a comorbid condition and that this negatively influences survival. In the present study, age, male gender and type of operation were not identified as risk factors, and although perioperative chemotherapy and radiotherapy may impact anastomosis site healing, they were not found to be related to stoma permanence. Furthermore, 52.2% of the patients in the NRG, but only 28.6% in the RG, had a comorbidity. Postoperative chemotherapy and radiotherapy for rectal cancer were delayed in patients with severe comorbid conditions, and this could have increased the risk of recurrence or systemic metastasis. We regarded 4-6 months after surgery as proper timing for stoma closure because postoperative adjuvant therapy was performed during that period. In the present study, the mean time between the main surgery and ileostomy closure was 7.4 months, which is comparable to previous studies [823]. Anastomosis-related complications are the main risk factor of a stoma nonreversal [7810]. Seo et al. [9] reported that anastomotic complications were a major risk factor of an early permanent stoma (within one year postoperatively) and the second most common cause of a late permanent stoma (>2 years postoperatively). The rate of a permanent stoma in patients who underwent colorectal surgery was reported to range from 18% to 25% [810151617]. In this study, in 22.3% of the patients (30/135) that underwent an ileostomy, the stoma was irreversible, and these patients included seven that required stoma reconstruction after ileostomy closure and 23 nonreversal patients. The rate of irreversibility in patients with stage IV cancer has been reported to be 30%, which compares with 3% in stages 0 to III [24]. Furthermore, the proportion of stage IV patients with a permanent stoma is high (66.7%) [8]. In the present study, stage IV was found to be an independent risk factor of a nonreversal stoma, and 47.8% of stage IV patients (11/23) had a permanent stoma. Local recurrence during follow-up period is an established risk factor of a nonreversal ileostomy. Local recurrence is an already known cause for a permanent stoma [89101921], and we found local recurrence rates in the RG and the NRG of 0.9% and 8.7%, respectively. In addition to anastomotic leakage or fistula, advanced cancer (stage IV), and local recurrence, we also found comorbidity to be an independent risk factor of a nonreversal stoma.
In the present study, the actual causes of ileostomy nonreversal were distant metastasis, poor anal sphincter tone, local recurrence, anastomotic leakage, radiation proctitis, and patient refusal. Poor patient general condition was attributed to comorbidities and to continuous chemotherapy and radiotherapy undertaken to reduce cancer progression. Four patients refused to undergo ileostomy repair due to an advanced age and economic status. In addition, one should bear in mind that poor anal sphincter function after sphincter-saving surgery for rectal cancer results in stoma irreversibility.
The present study is limited by its small size and nonrandomized, retrospective design. Furthermore, we did not evaluate the effects of preoperative or postoperative CCRT on stoma reversibility. Nevertheless, several interesting trends were detected.
In conclusion, postoperative complications such as anastomotic leakage or fistula, advanced cancer (stage IV), local recurrence, and comorbidities were identified as risk factors of a nonreversal ileostomy. We advise that these factors be considered when drafting prudential guidelines for stoma closure.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.