Efficacy of Immunohistochemical Staining in Differentiating a Squamous Cell Carcinoma in Poorly Differentiated Rectal Cancer: Two Case Reports
Article information
Abstract
A rectal carcinoma, including primary an adenosquamous and a squamous cell carcinoma (SCC), is a very rare disease, accounting for 0.025% to 0.20% of all large-bowel malignant tumors. Because SCCs have a higher mortality than adenosquamous carcinomas, determining whether the primary rectal cancer exhibits an adenomatous component or a squamous component is important. While differentiating between these 2 components, especially in poorly differentiated rectal cancer, is difficult, specific immunohistochemical stains enable accurate diagnoses. Here, we report the use of immunohistochemical stains to distinguish between the adenomatous and the squamous components in 2 patients with low rectal cancer, a 58-year-old man and a 73-year-old woman, who were initially diagnosed using the histopathologic results for a poorly differentiated carcinoma. These data suggest that using these immunohistochemical stains will help to accurately diagnose the type of rectal cancer, especially for poorly differentiated carcinomas, and will provide important information to determine the proper treatment for the patient.
INTRODUCTION
Most rectal malignancies are adenocarcinomas, which are primarily treated surgically, with selective use of neoadjuvant or adjuvant chemo-radiation for more locally advanced disease. Rectal primary adenosquamous carcinoma or squamous cell carcinoma (SCC) is very rare, with an incidence of 0.025% to 0.20% among all large bowel malignant tumors [1]. Most cases of rectal SCC are actually anal SCC with proximal extension into the rectum, which is different from primary SCC of the rectum. The estimated incidence of primary rectal SCC is approximately 0.01%–0.025% of all colorectal neoplasms [2]. William et al. [3] established the following criteria to define SCC of rectal origin: (1) metastasis from other organs to the rectum must be ruled out; (2) there is an absence of anal involvement with SCC; and (3) an anal SCC-lined fistula with the rectum must be ruled out. Various treatment options are being used to manage rectal adenosquamous carcinoma or SCC, but there is still difficulty in determining appropriate treatments. While surgery is considered the gold-standard therapy, the combination of radiotherapy and chemotherapy is an effective alternative therapy [4]. Thus, differentiating among adenocarcinoma, adenosquamous carcinoma, and SCC is a critical issue. Using immunohistochemical stains may be an effective method to differentiate these pathologies. A study from Memorial Sloan Kettering Cancer Center showed that immunohistochemical stains are useful in distinguishing between adenosquamous carcinoma and SCC of the rectum, especially in poorly differentiated cancer [5]. Therefore, we describe 2 cases of poorly differentiated rectal cancer, in which various immunohistochemical stains reveal the final pathology containing either an adenomatous component or a squamous component.
CASE REPORTS
Case 1
A 58-year-old man presented with lower rectal swelling, bleeding, and anemia for 3 years. He was a diabetic patient with no family history of colorectal cancer. A colonoscopy revealed a rectal mass 4 cm above the anal verge (AV), and histopathology from another hospital showed a poorly differentiated carcinoma. His serum carcinoembryonic antigen (CEA) level was 4.06 ng/mL. Pelvic magnetic resonance imaging (MRI) and computed tomography (CT) of the abdomen and the pelvis showed a fungating mass in the lower rectum extending to the anal canal, with multiple lymph nodes (LNs) in the perirectal and right pelvic side wall without any other evidence of distant metastasis (Fig. 1A). Whole-body positron emission tomography-CT showed intense fluorodeoxyglucose uptake in the LNs at the perirectal and both iliac chains without any suggestion of distant metastasis.
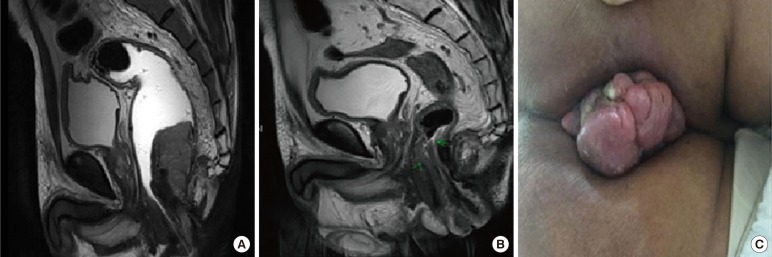
(A) Pelvic magnetic resonance imaging (MRI) revealed a 6.1-cm fungating mass involving the lower rectum (4 cm from the anal verge). Multiple regional lymph node metastases were suspected. (B) Post concurrent chemo-radiotherapy MRI shows a decrease in the size of the pedunculated mass protruding into the anal canal (from 6 cm to 4.7 cm in size) with no extramural extension. (C) Encircled prolapsed anal mass.
To differentiate the type of cancer, the patient underwent rebiopsy. This revealed a poorly differentiated carcinoma, suggestive of a squamous-cell type. Therefore, additional immunohistochemical staining and a special stain analysis were performed. From the stains, p40 was positive and CK20, CEA, and HMB45 were negative in the tumor cells. Based on the diagnosis of a SCC, the patient started concurrent chemo-radiotherapy (CCRT) for 6 weeks, based on a regimen of fluorouracil and mitomycin C with 50.4 Gy. One month after CCRT, follow-up rectal MRI and abdominal and pelvic CT were performed. The scans showed a reduction in the size of the pedunculated mass protruding into the anal canal with no extramural extension. Also noted was a reduction in the sizes of the perirectal and the right iliac LNs (Fig. 1B). However, the patient still presented with a prolapsing anal mass (Fig. 1C).
After a multidisciplinary team approach, the patient underwent excision of the prolapsed lesion to confirm the pathology, which showed no residual tumor with nonspecific inflammation. Two months later, rectal MRI showed a new 8.4 × 3.1-cm lobulating mass that involved the entire anal canal and low rectum and was suspected to be a recurrent tumor. A rebiopsy was performed, and the pathology report showed a poorly differentiated carcinoma expressing p63 and p40, consistent with a SCC, while CDX2 and HMB45 were negative in the tumor cells (Table 1). The patient had an abdominoperineal resection, and the pathology report showed Mandard grade IV regression with a poorly differentiated carcinoma and squamous differentiation, classified as ypT3N0M0. All of the margins were free, but lymphovascular invasion (LVI) and perineural invasion were seen. The permanent immunohistochemical staining results showed positive p40 with weakly positive p63, and negative CDX2 staining (Fig. 2). The patient was administered 5-fluorouracil and cisplatin chemotherapy after surgery, and he is now on his third chemotherapy cycle.
Case 2
A 73-year-old woman presented with a 3-month history of changes in stool habit and stool caliber. She had a positive stool occult blood test, and her serum CEA level was 1.69 ng/mL. Colonoscopy showed 2 masses; one was 1 cm from the AV, and the other was 8.5 cm from the AV (Fig. 3A). The pathology report from another hospital indicated a diagnosis of a poorly differentiated carcinoma. Rectal MRI and CT of the abdomen and the pelvis showed a fungating mass in the midrectum, with perirectal fat infiltration and multiple enlarged LNs in the mesorectum. No pelvic side wall or para-aortic lymphadenopathy was indicated (Fig. 3B), and chest CT showed no evidence of intrathoracic metastasis. Rebiopsy was performed for suspected lesions, and the pathology report showed a fragment of scattered tumor cells with p63-positive nuclei, consistent with a SCC.

(A) Colonoscopy showed two masses, the first being 1 cm and the second 8.5 cm from the anal verge. (B) Rectal magnetic resonance imaging (MRI) showing a fungating mass in the mid rectum 8.5 cm from the anal verge with perirectal fat infiltration and multiple enlarged lymph nodes in the mesorectum. (C) Rectal MRI after concurrent chemo-radiotherapy showed a decrease in the tumor size with still a positive circumferential resection margin at 8 o'clock direction.
Because the disease was considered as T1 anal cancer and T3 rectal cancer positive for a circumferential resection margin (CRM), a multidisciplinary team decided to start neoadjuvant CCRT. After 4 weeks of Xeloda-based 50.4-Gy CCRT, follow-up rectal MRI and CT of the abdomen and the pelvis showed decreased tumor volume with mesorectal infiltration, but the scans were still positive for CRM at the 8 o'clock position with no significant LNs in the pelvic cavity or evidence of distant metastasis (Fig. 3C). Ten weeks after CCRT, the patient underwent a laparoscopic-assisted low anterior resection with double stapling for rectal cancer and a transanal excision for anal cancer (Fig. 4). The pathological diagnosis after surgery showed post-CCRT status with fibrosis outgrowing the residual cancer with a main diagnosis of a poorly differentiated adenosquamous carcinoma. LVI was positive, and an ulcerative 2.5 × 1.5-cm mass with a classification of ypT3N0M0 was seen.
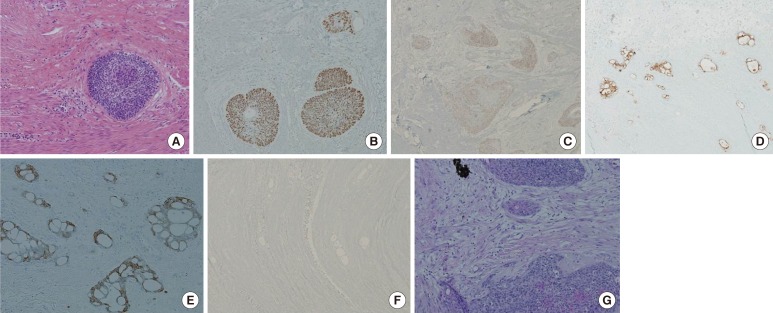
(A) H&E, ×100, (B) positive in squamous cell carcinomatous component (P40, ×100), (C) positive in squamous cell carcinomatous component (P63, ×40), (D) positive in adenocarcinomatous component (CEA, ×40), (E) positive in adenocarcinomatous component (CK20, ×100), (F) negative in tumor cells (CDX2, ×40), and (G) focal mucin deposits in tumor cells (D-PAS, ×100).
The immunohistochemical stain results showed positive for p40 and p63, suggesting a squamous cell carcinomatous component. Additionally, CEA and CK20 were positive for the adenocarcinomatous component, and CDX2 was negative in the tumor cells. D-PAS stain revealed focal mucin deposits in the tumor cells (Table 1). A follow-up CT scan of the abdomen and the pelvis one month after the surgery showed no evidence of tumor recurrence or metastasis, and the patient started on 4 cycles of FP (5-fluorouracil and cisplatin) chemotherapy. The FP chemotherapy has been completed, and the patient is being followed up at an outpatient clinic without any recurrence.
DISCUSSION
Colorectal cancer is a common disease that usually presents with an adenocarcinoma histology. However, only a few reports in the literature are concerned with colorectal SCCs. The etiology of a SCC is uncertain. According to Balfour, a true SCC of the colon and rectum does not exist, and this disease might represent metastasis from other sites or squamous degeneration of a pre-existing adenocarcinoma. However, this opinion is not widely accepted [6]. Several other theories have attempted to explain the histopathogenesis of these tumors. These theories include (1) the presence of embryologic nests of ectodermal cells in the colonic mucosa; (2) the occurrence of squamous metaplasia of the intestinal mucosa, specifically in colonic adenomas; (3) the capability of pluripotent stem cells of endodermal origin to transform into an adenocarcinoma, a SCC, or both; and (4) possible cellular degeneration within an existing adenocarcinoma secondary to an abnormal mucosa stimulus such as ulcerative colitis or radiation [17]. Because defining an adenocarcinoma, an adenosquamous carcinoma or a SCC, is difficult in poorly differentiated cancer, several immunohistochemical markers are used to distinguish squamous-cell disease from an adenocarcinoma (Table 2).
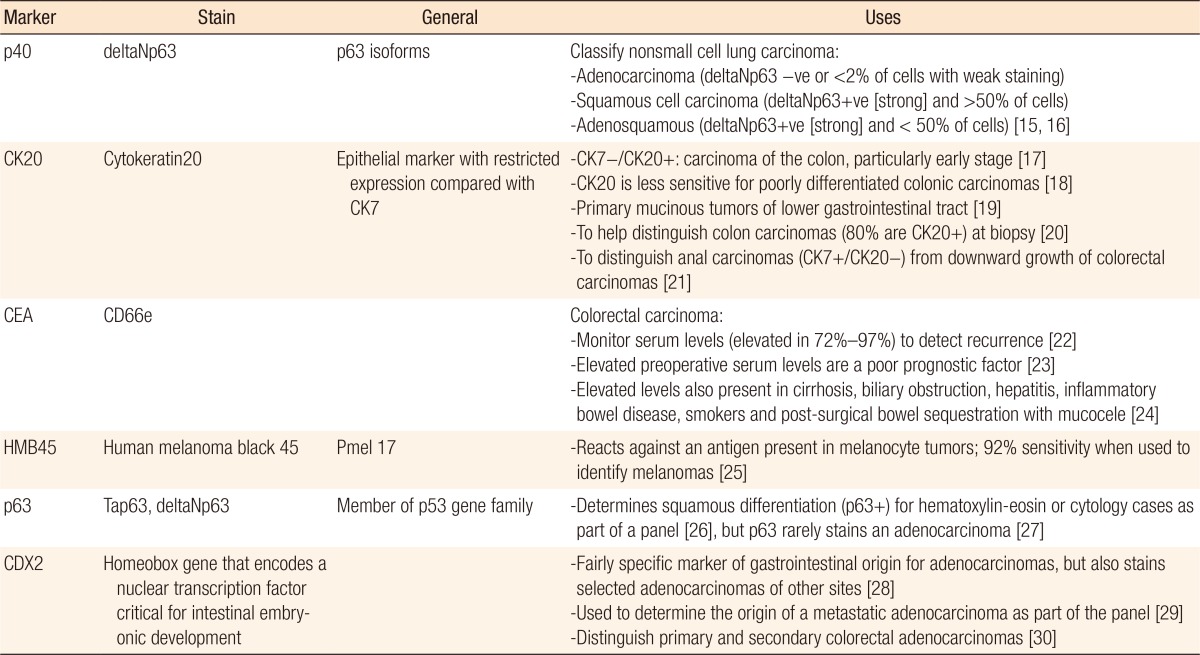
Immunohistochemical stain markers used to distinguish between the adenomatous and squamous components
Hematoxylin-eosin (H&E) staining is first applied to detect whether the adenomatous morphology is present. Following the stain analysis, if clarifying the type is still difficult, extra immunohistochemical stains should be used. In this report, both cases required extra staining to confirm the diagnosis because the primary result was only a poorly differentiated carcinoma. p40 and p63 are important markers for discriminating squamous cell components from adenocarcinoma components because these markers are positive in SCCs. p63 is p53 homolog nonspecific antibody, subdivided by TAp63 and deltaNp63, which is called p40, so the expression of deltaNp63, as well as that of p63, is compared with both the clinical and the pathological factors and prognosis [8].
In the first case, we also stained with HMB45 to distinguish this cancer, which presented very poor differentiation in the anus, from a malignant melanoma. Furthermore, because H&E staining suggested an adenosquamous carcinoma in the second case study, D-PAS, which can be derived from an adenocarcinoma, was stained only in the second case. In both cases, additional staining for the expressions of CEA, CK20, and CDX2 was performed. These markers are positive in an adenocarcinoma, possibly aiding in the discrimination of the adenomatous component from the primary cancer.
Early discrimination of a rectal adenocarcinoma from an adenosquamous carcinoma or a SCC is critical because of differences among prognoses. For instance, the prognosis for a rectal SCC is worse than that of other parts of the colon [9]. A preoperative diagnosis is difficult because the clinical presentation of a SCC is similar to that of an adenocarcinoma; symptoms include abdominal pain, anemia, weight loss, rectal bleeding, and tenesmus [1011]. Thus, with the help of immunohistochemical staining analysis, discriminating among a rectal adenocarcinoma, adenosquamous disease, and a SCC is possible. The squamous epithelial components have more invasive tendencies than the glandular components [7], and the survival rates are lower in the epithelial components [1011]. Furthermore, in cases of LN metastasis, the prognosis for an adenosquamous carcinoma or a SCC is much worse than that for an adenocarcinoma alone [6]. The 5-year survival rate for a node-positive adenosquamous carcinoma or a SCC is 23% compared with 85% for node-negative cases. The overall 5-year survival rate for an adenosquamous carcinoma or a SCC is 31% compared with 66% for an adenocarcinoma [12].
Overall, surgical resection is traditionally considered the most effective treatment not only to cure the patient but also to confirm the pathology. Some researchers have suggested that chemoradiation may be an effective treatment, or even consider it a definitive treatment, reserving surgery for patients for whom chemoradiation treatment has failed [1013]. The low acute toxicity of chemoradiation and the rare incidence of long-term toxicity, reported as symptomatic rectal stricture, are reasons to consider treatments other than surgery, which has the risks of morbidity and mortality [14]. Thus, chemotherapy or radiotherapy should be considered, especially in node-positive cases, although further investigation is needed regarding whether to treat patients with chemotherapy or radiotherapy alone or to treat them with a combination of these therapies.
In summary, compared to an adenocarcinoma, an adenosquamous carcinoma and a SCC exhibit both poor prognoses and difficulties in determining treatment plans. Using several immunohistochemical stains, we were able to distinguish squamous components from adenomatous components, especially in patients with poorly differentiated cancer. By differentiating between an adenosquamous carcinoma and a SCC, physicians may employ more effective treatments for these rare malignancies.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.

