The First Year After Colorectal Surgery in the Elderly
Article information
Abstract
Purpose
Surgery for colorectal malignancy is increasingly being performed in the elderly. Little is known about the impact of complications on late mortality. This study aimed to analyze whether a complicated postoperative course affects the 1-year survival in elderly patients.
Methods
All consecutive patients older than 75 years of age who underwent colorectal cancer surgery between January 2009 and April 2013 were included in this study. The main outcome was mortality at 1 year after surgery. Logistic regression analyses were performed to determine risk factors for a poor outcome (mortality) after survival of the early postoperative course of surgery at 1-year follow-up. Patients who died within 30 days postoperatively were excluded from analysis.
Results
The early mortality rate was 6.3% (n = 15), and 2 patients died during follow-up as a result of complications after a second surgery. A total of 223 patients survived the perioperative period and were included in this study. Twenty-two patients (9.9%) died during the first year of follow-up. Stage IV disease (P = 0.002), complications of primary surgery (P = 0.016), and comorbidity (P = 0.050) were risk factors for 1-year mortality. Intensive care unit stay, reoperation and readmission were not associated with a worse 1-year outcome.
Conclusion
Elderly patients with stage IV disease at the time of surgery, comorbidity, and postoperative complications are at risk for mortality during the first year after surgery. A patient-tailored approach with special attention to perioperative care should be considered in the elderly.
INTRODUCTION
With aging of the population, colorectal surgery for cancer in older patients is increasingly being performed [1]. The surgical treatment of elderly patients with colorectal malignancies is challenging [2]. Two systematic reviews report lower overall survival rates compared with younger patients [34]. Understandably, the highest mortality rate in the elderly occurs during the early postoperative period [56], but cancer-related survival is equal in older and younger patients when patients survive the first year after colorectal surgery [57]. However, little is known about the survival and the readmission rates during the first year after surgery and about whether the outpatient course is determined by the early postoperative in-hospital course. The aim of this study was to evaluate the course of recovery immediately after the postoperative period and to investigate the influence of this early postoperative course on the outcome after 1 year. This knowledge may allow a patient-tailored approach and shared decision-making during hospital admission if complications of surgery occur.
METHODS
Patients aged 75 years or older who had undergone colorectal cancer surgery at the St Antonius Hospital, Nieuwegein, The Netherlands, between January 2009 and April 2013 were enrolled in a retrospective database. Data on the postoperative course, including postoperative complications (i.e., surgical and nonsurgical complications within 30 days after surgery, during admission, or during readmission within 30 days), early mortality (mortality within 30 days postoperatively or in-hospital), mortality during follow-up (within 1 year following surgery), reoperations, intensive care unit (ICU) stay, and readmissions, were evaluated. Comorbidity was categorized using the American Society of Anesthesiologists physical status (ASA PS) classification and the Charlson Comorbidity Index [89]. The Common Terminology Criteria for Adverse Events were used to define postoperative complications [10], and the Clavien-Dindo classification of surgical complications was used to classify the severity of complications [111213].
Data are presented as number of patients with percentage or as median with interquartile range (IQR). Patients who died during the first 30 days after surgery or in-hospital (early mortality) were excluded from the analysis to determine only the variables contributing to a worse 1-year outcome in ‘survivors of the surgery’.Differences between groups were tested with the chi-square test (for binomial outcomes). A logistic regression analysis (enter method) was used to determine independent risk factors for 1-year mortality in survivors of surgery, including all variables with a P-value inferior to 0.05 in the univariate analysis. A P-value ≤ 0.05 (2-tailed) was considered significant. Data were analysed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA).
RESULTS
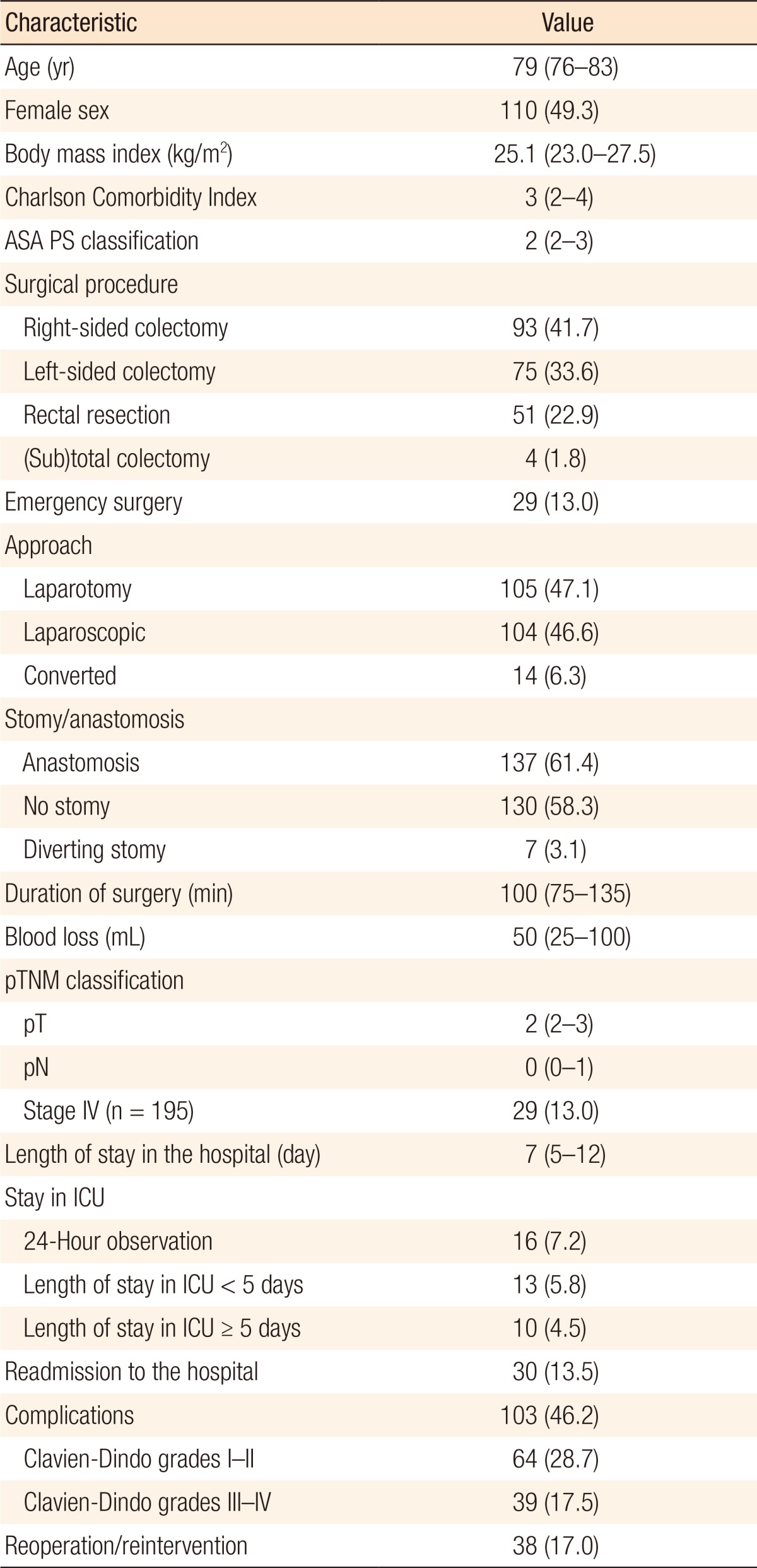
A total of 256 patients were included. Of these patients, follow-up was incomplete in 16 patients (6.3%). These patients were excluded from analysis. The early mortality rate was 6.3% (n = 15) as a results of surgical (n = 5) and nonsurgical (n = 10) postoperative complications. A total of 225 survived the early postoperative period after colorectal surgery. Two patients died during follow-up due to complications after a second surgical procedure (1 stoma reversal and 1 adhesiolysis with partial small bowel resection for ileus). The remaining 223 patients were included in the analysis: 113 male (50.7%) and 110 female patients (49.3%) with a median age of 79 years (IQR, 76–83 years). Baseline and postoperative characteristics are summarized in Table 1.
During the first year after surgery, 22 of the 223 included patients (9.9%) died due to progressive cardiopulmonary morbidity (n = 8), secondary (lung) cancer (n = 1), and progression of cancer with distant (organ) metastases or progression of clinical deterioration as a result of the cancer (n = 13). After 1 year, 201 of the 223 patients (90.1%) were still alive.
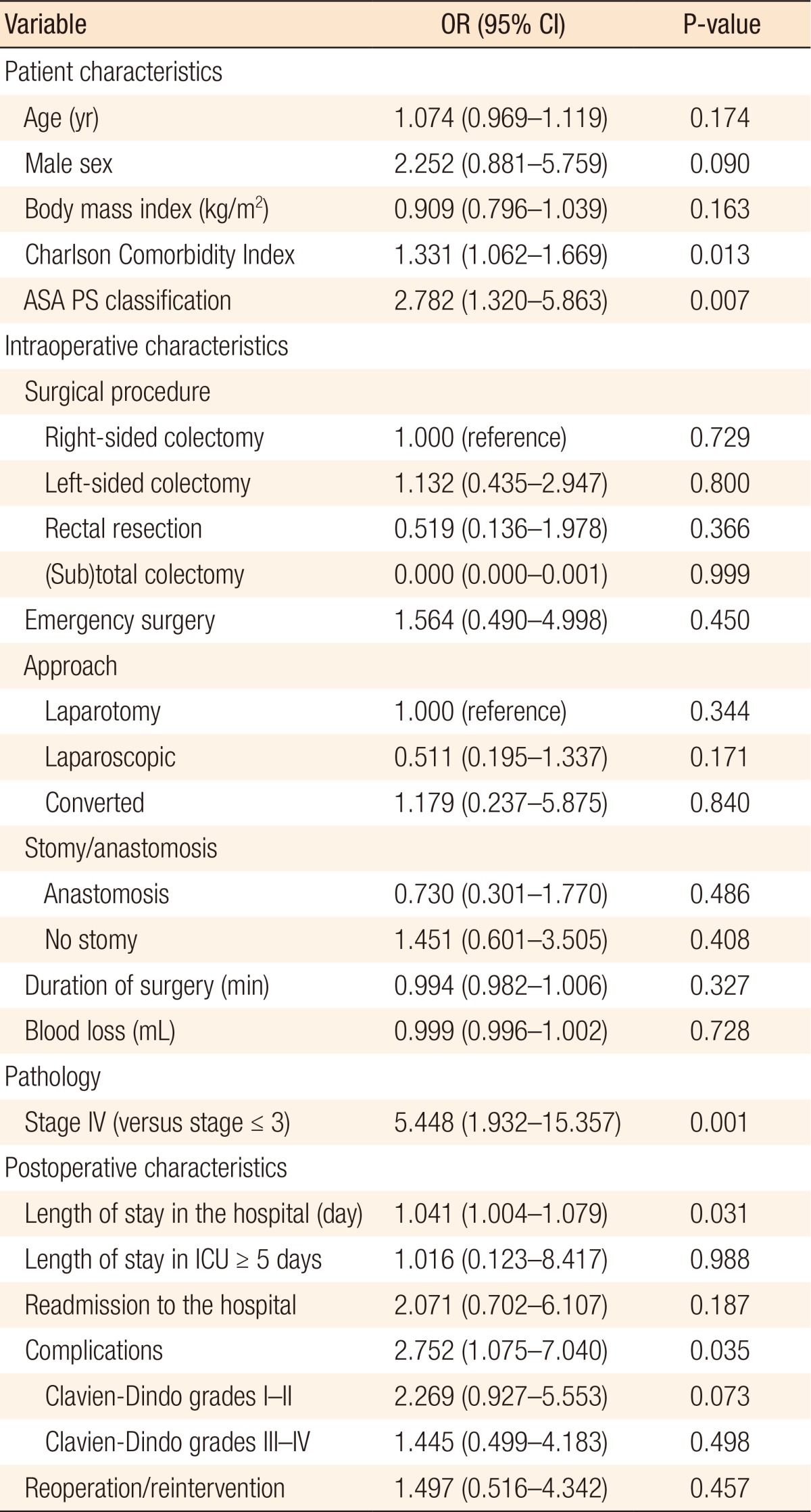
Postoperative complications after initial colorectal surgery occurred in 103 patients (46.2%) and were mostly graded as Clavien-Dindo grades I–II (Table 1). Thirty-eight patients needed reoperation due to complications (Clavien-Dindo grades III–IV). The occurrence of postoperative complications was associated with death during the first year of follow-up. Of patients with postoperative complications, 68.2% (n = 15) died after discharge during the first year versus 31.8% of the patients who died and had not suffered from complications (n = 7) (P = 0.041). The grade of the complications (Clavien-Dindo grade ≥III) did not influence the risk of death after discharge nor did the need for reoperation (P = 0.498 and P = 0.457, respectively) (Table 2).
A total of 13 patients (5.8%) were admitted to the ICU for less than five days as a result of postoperative complications, and 10 patients (4.5%) required more than five days of ICU care (Table 2). However, ICU stay (short or long) was not associated with mortality during follow-up (P = 0.988). Thirty patients (13.5%) were readmitted to the hospital within 30 days following surgery. After readmission, 83.3% of the patients survived the first year. Readmission was not associated with worse 1-year survival (P = 0.187) (Table 2).
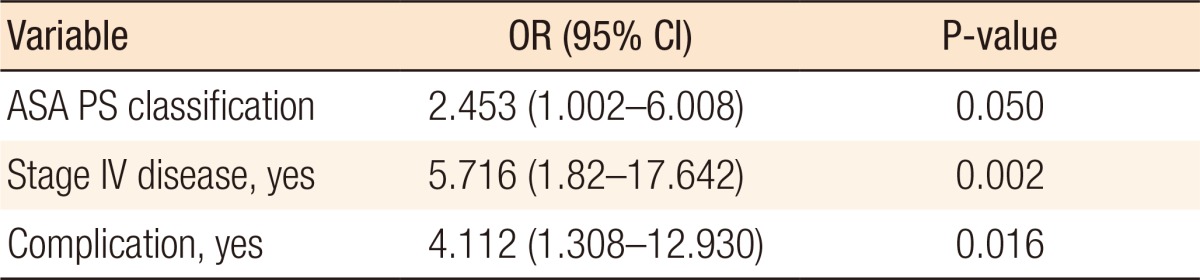
All survivors of primary surgery were added to the logistic regression analysis with the outcome being mortality at 1 year. In the univariate analysis, ASA PS classification, Charlson Comorbidity Index, stage IV disease, length of stay in the hospital, and postoperative complications were associated with mortality at 1 year (Table 2). ICU stays of more than 5 days, reoperations, and readmissions were not associated with 1-year mortality. Variables added to the multivariate analysis were ASA PS classification, stage IV disease, and postoperative complications; Charlson Comorbidity Index and length of stay in the hospital were excluded from the analysis because of their high correlations with the other variables. For patients who survived the primary surgery, all three included parameters were independently associated with mortality at 1 year with odds ratios (ORs) of 5.716 (95% confidence interval [CI], 1.82–17.642) (P = 0.002) for stage IV disease, 4.112 (95% CI, 1.308–12.930) (P = 0.016) for postoperative complications, and 2.453 (95% CI, 1.002–6.008) (P = 0.050) for ASA PS classification (Table 3).
DISCUSSION
This is the first study to demonstrate that 10% of the elderly patients who undergo colorectal cancer surgery are likely to die within 1 year after discharge. Tumor stage, postoperative complications, and ASA PS classification were associated with worse 1-year outcome in survivors of the early postoperative period. Patients who died during the early postoperative period all had complications of high degree, but for those who survived this early period, the 1-year survivals were equal for patients with high and those with low degrees of complications.
The length of ICU stay was not associated with a worse outcome at 1 year. This indicates that if a patient survives a prolonged ICU stay, the prognosis for the next year is comparable to that for other patients who had not had such an experience. However, no information about quality of life (QoL) after (prolonged) ICU stay is known. A study by Montuclard et al. [14] concluded that prolonged ICU stay (>30 days) in elderly patients (≥70 years old) was associated with an acceptable QoL, but emerging evidence suggests that (prolonged) ICU stay is associated with a decreased quality of life known as the postintensive care syndrome [151617].
Dekker et al. [5] demonstrated slightly higher 1-year mortality rates of 20.1%–23.2% in elderly (≥75 years) colorectal cancer patients. Factors that were significantly associated with an impaired 1-year outcome in their study were male sex (in rectal cancer surgery), increased age, and tumor grade (in both colon and rectal cancer surgery). When patients survived the first year after surgery, survival was comparable for both older and younger patients for both colon and rectal cancer surgery [5]. In a study among patients of 70 years or older who underwent noncardiac surgery, postoperative pulmonary and renal complications also proved to be independently associated with a decreased long-term survival (hazard ratios of 2.41 and 6.07 (P < 0.001), respectively) [18].
A study demonstrated that elderly patients were more likely to be readmitted to the hospital compared with younger patients [19]. Furthermore, readmission was found to be strongly associated with a higher 1-year mortality rate (adjusted OR, 2.44; 95% CI, 2.25–2.65) [20]. The reasons for readmission were comparable with those in our study, but readmission was not associated with a worse 1-year outcome in our study, which only included elderly (unadjusted OR, 2.07; 95% CI, 0.70–6.11) (P = 0.187).
The surgical treatment of colorectal malignancies in the aging population remains a formidable challenge, and the chances of a successful outcome with a good quality of life over the remaining life span need to be weighed against the risk of potential complications with a detrimental outcome. Present-day informed-consent procedures and shared decision-making with patients and relatives require an optimal knowledge of the factors influencing the outcome of surgical procedures. The management of the older patient requires a stepwise approach at different stages. In the preoperative stage, an optimal risk assessment is necessary either to decide to exclude the patient from surgery based on morbidity profiles or to define the level of needed care tailored to the patient's clinical and physical status. An individualized rehabilitation program should guarantee an optimization of the preoperative nutritional and cardiopulmonary status. In the perioperative stage, state-of-the-art surgical and anesthesiological techniques are obligatory, and every effort should be put into the prevention, early detection, and containment of perioperative complications. Early identification of patients at risk for an adverse perioperative course is of paramount importance. In patients with a considerable comorbidity, a multidisciplinary approach may be opted for to optimize the preoperative condition and to prevent (comorbidity-related) postoperative complications. A patient-tailored approach with special attention to perioperative care is essential in the elderly.
In this study, we demonstrated that once the patient survives colorectal surgery, albeit after reoperation or prolonged ICU care, survival during the first year after discharge is comparable to that of other patients without such experiences. We also demonstrated that the occurrence of complications (which are mainly cardiopulmonary) after surgery is a predictor of increased risk of death. Thus, an elderly patient suffering from postoperative complications after colorectal surgery should be the focus of special attention in order to provide the best chance for survival.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.