Difference in Tumor Area as a Predictor of a Pathological Complete Response for Patients With Locally Advanced Rectal Cancer
Article information
Abstract
Purpose
This study was conducted to discover the clinical factors that can predict pathologically complete remission (pCR) after neoadjuvant chemoradiotherapy (CRT), so that those factors may help in deciding on a treatment program for patients with locally advanced rectal cancer.
Methods
A total of 137 patients with locally advanced rectal cancer were retrospectively enrolled in this study, and data were collected retrospectively. The patients had undergone a total mesorectal excision after neoadjuvant CRT. Histologic response was categorized as pCR vs. non-pCR. The tumor area was defined as (tumor length) × (maximum tumor depth). The difference in tumor area was defined as pre-CRT tumor area – post-CRT tumor area. Univariate and multivariate logistic regression analyses were conducted to find the factors affecting pCR. A P-value < 0.05 was considered significant.
Results
Twenty-three patients (16.8%) achieved pCR. On the univariate analysis, endoscopic tumor circumferential rate <50%, low pre-CRT T & N stage, low post-CRT T & N stage, small pretreatment tumor area, and large difference in tumor area before and after neoadjuvant CRT were predictive factors of pCR. A multivariate analysis found that only the difference in tumor area before and after neoadjuvant CRT was an independent predictor of pCR (P < 0.001).
Conclusion
The difference in tumor area, as determined using radiologic tools, before and after neoadjuvant CRT may be important predictor of pCR. This clinical factor may help surgeons to determine which patients who received neoadjuvant CRT for locally advanced rectal cancer should undergo surgery.
INTRODUCTION
In patients with locally advanced rectal cancer, the standard treatment is a total mesorectal excision at 4–8 weeks after neoadjuvant chemoradiotherapy (CRT). Compared with postoperative CRT, preoperative CRT has a relatively low toxicity, reduces the local recurrence rate, and enhances the chance of preserving the anal sphincter; nevertheless, it has no effect on long-term survival [12]. Of the patients who undergo neoadjuvant CRT, 10%–30% show a pathologically complete response (pCR) [34567]. In these patients, the oncological outcome is better than it is in patients who do not achieve a pCR [48910].
In this regard, Habr-Gama et al. [11] suggested a “watch-and-wait” strategy, in which patients who show a clinical complete response undergo close clinical and radiological follow-up rather than immediate surgery. Recent studies reported that the “watch-and-wait” strategy resulted in prognoses similar to those achieved with neoadjuvant CRT and surgery [12131415]. If pCRs can be predicted preoperatively, then morbidities, such as postoperative complications, permanent stoma, sexual dysfunction, and urinary dysfunction, may be avoided. Unfortunately, no clinical data, including endoscopic and radiological studies, can accurately predict a pCR [16171819]. For these reasons, this study examined the clinical predictors of a pCR after neoadjuvant CRT to help determine a treatment program for the management of patients with locally advanced rectal cancer.
METHODS
This retrospective study enrolled 137 patients who had undergone a total mesorectal excision after neoadjuvant CRT at the Department of Surgery, Inje University Busan Paik Hospital, between March 2000 and December 2015. All patients had a pathologically confirmed adenocarcinoma based on pretreatment colonoscopy. The clinical stage was established using pelvis magnetic resonance imaging (MRI), but for those who had not undergone MRI, abdomen and pelvis computed tomography (APCT) was used to establish the clinical stage. The sensitivity and the specificity of pelvis MRI are well known to be better than those of APCT in T & N staging of rectal cancer, but some patients refuse to undergo pelvis MRI because of their economic status. Therefore, every patient had undergone pre/post CRT APCT, but only about half had undergone MRI. This study included patients whose rectal cancers were located within 10 cm above the anal verge and were in stage T3 or T4 and/or who were lymph-node positive. Those with distant metastases or other pretreatment malignancies were excluded. The Institutional Review Board of Inje University Busan Paik Hospital approved this study (approval number: 16-0237). Formal consent of the patients is not required for this type of study.
External beam radiotherapy was performed with the patient in the prone position using a belly board. A 3-field technique and a 3-dimensional informal technique were used in all patients. The patients received a total dose of 50.4 Gy, at a once-daily dose of 1.8 Gy. All patients were treated with 1.8-Gy fractions. All patients were treated concurrently with either 2 cycles of bolus infusion 5-fluoruracil (5-FU) at 425 mg/m2 per day, 5 times weekly, every 4 weeks or with capecitabine at 825 mg/m2 twice a day, 5 days per week for 6 weeks. Among the patients assigned to preoperative treatment, surgery was scheduled to take place 6 weeks after the completion of CRT. Four cycles of bolus infusion 5-FU at 425 mg/m2 per day, 5 times weekly, every 4 weeks were started 4 weeks after surgery.
One colonoscopist and 1 radiologist retrospectively reviewed the colonoscopic findings and the results of the imaging studies (APCT & MRI). This was done to minimize any possible bias because interpretation of the images can be subjective and may vary between doctors. The colonoscopist measured the endoscopic circumferential rate and classified the tumor's location and morphology, and the radiologist depicted the imaging studies (APCT & MRI). He measured pre CRT T & N stage, post CRT T & N stage, the tumor's area (tumor length × maximum tumor depth), and the difference in tumor area. The tumor area was measured using MRI in the presence of pre/post CRT pelvis MRI and using APCT in the absence of pre/post CRT pelvis MRI. (The difference in tumor area = pre CRT tumor area – post CRT tumor area).
All values are presented as means ± standard deviations for continuous variables and as numbers and percentages for discrete variables. We performed the t-test or the Wilcoxon rank sum test and the chi-square test or Fisher exact test, as appropriate. A receiver operating characteristic curve analysis was used in order to identify the optimal cutoff value of the difference in tumor area that could predict a pCR. Univariate and multivariate logistic regression analyses were conducted to identify the factors predictive of a pCR. The variables with P-values <0.05 in the univariate analyses were used in the multivariate analyses. A P-value <0.05 was considered significant. SAS ver. 9.3 (SAS Institute Inc., Cary, NC, USA) was used for the statistical analysis.
RESULTS
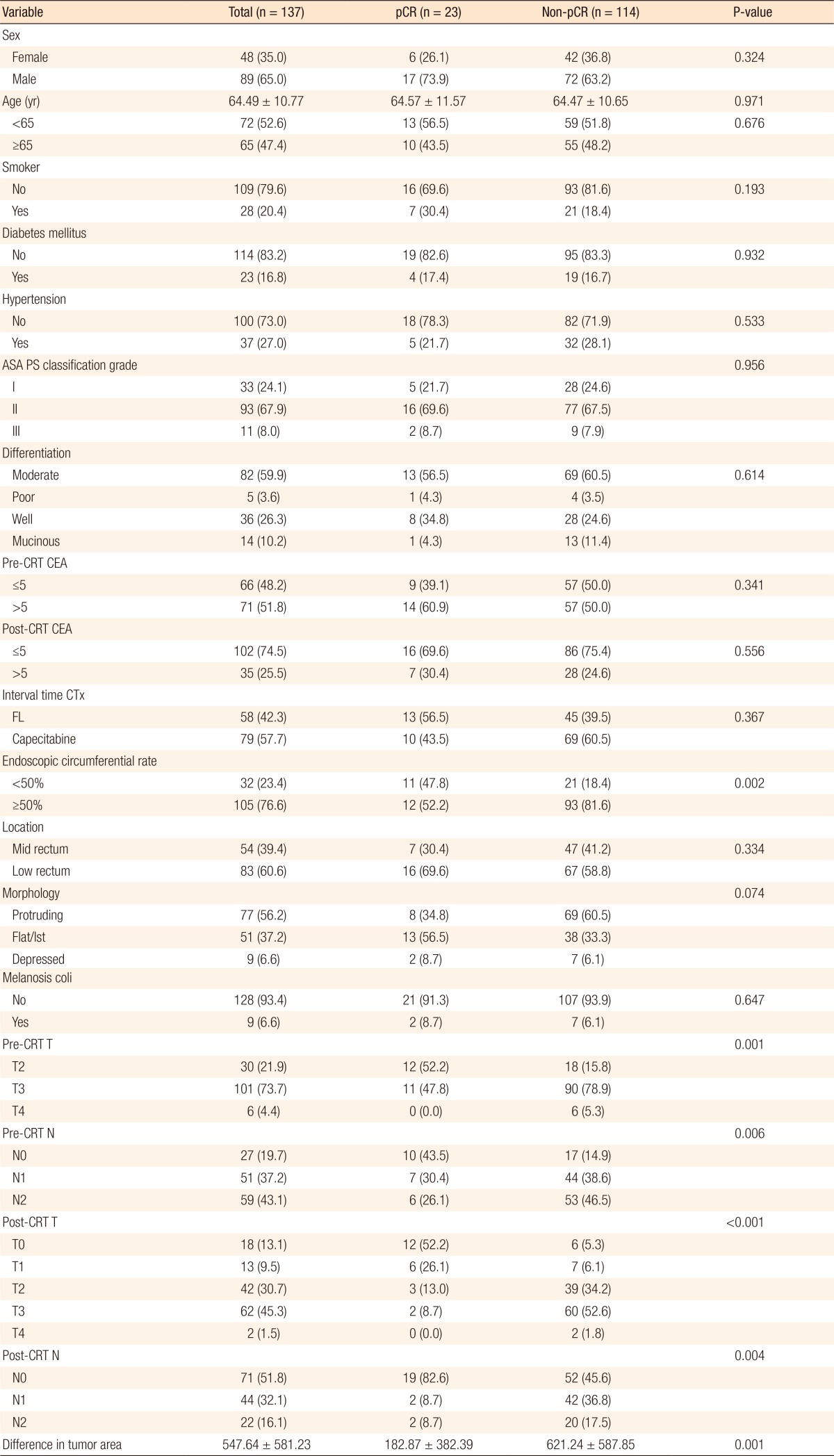
Table 1 summarizes the patients' demographics and the tumors' characteristics. Of the 137 patients who received neoadjuvant CRT, 23 (16.8%) achieved a pCR. The patients were divided into the pCR (n = 23) and the non-pCR (n = 114) groups for comparison. Patients with an endoscopic circumferential rate below 50% had a significantly higher pCR rate than did those with a rate over 50% (P < 0.001). Moreover, the lower the pre-CRT T (P = 0.001) and N (P = 0.001) stages and the post-CRT T (P < 0.001) and N (P = 0.004) stages were, the higher the pCR rate was. In addition, the smaller the tumor area (tumor length × maximum tumor depth) measured pre-CRT using CT or MRI was, the higher the pCR rate (P < 0.001) was (Table 1). No other significant differences between the 2 groups were noted.
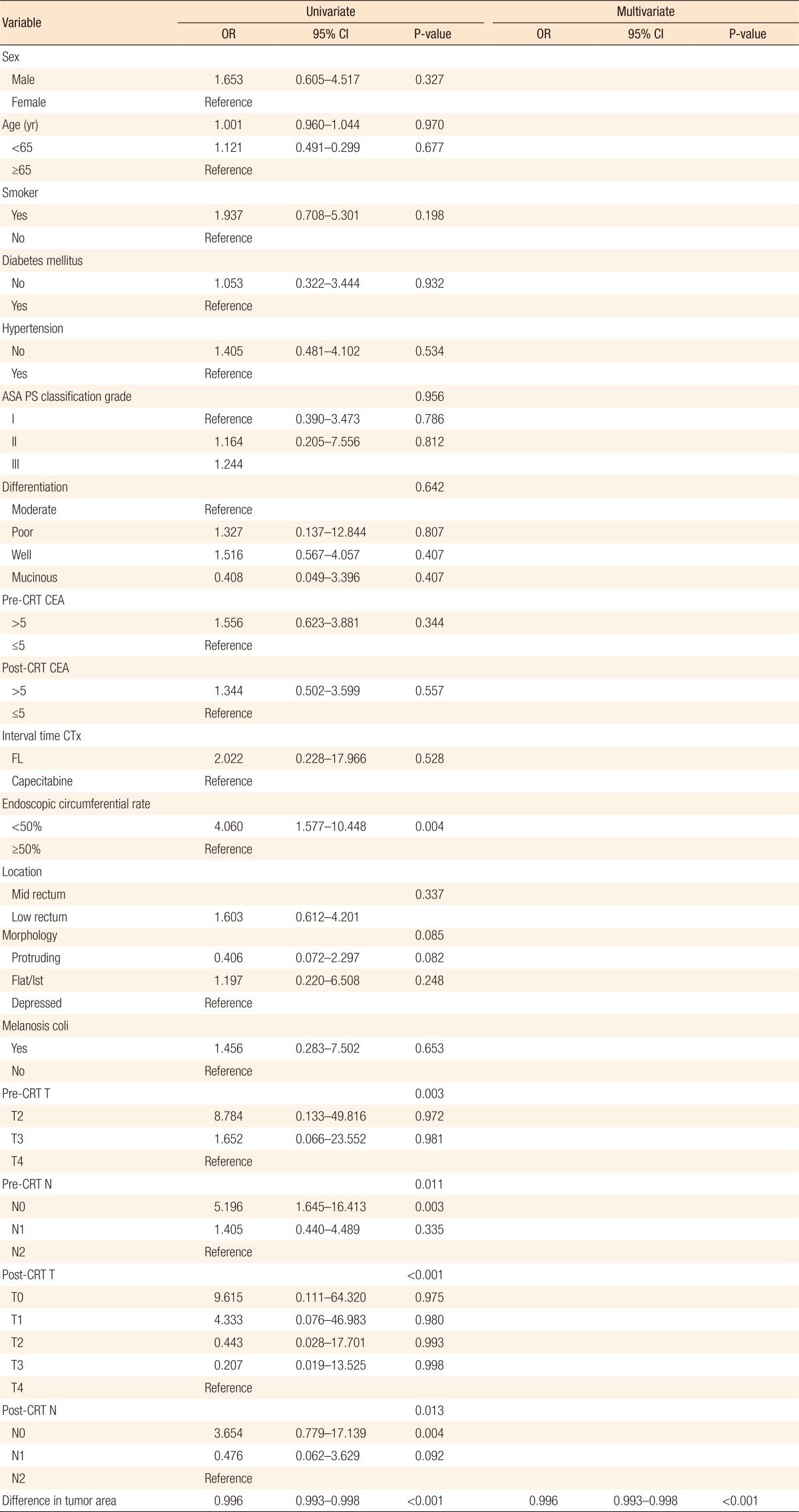
The optimal cutoff value of the difference in tumor area was 574. The sensitivity and the specificity were 13% and 64% (odds ratio [OR], 3.774; 95% confidence interval [CI], 1.049–13.364; P = 0.042). Fig. 1 shows the receiver operating characteristic (ROC) curves of the difference in tumor area to predict pathologically complete response. Table 2 summarizes the results of the univariate and the multivariate analyses. On the univariate analyses, an endoscopic tumor circumferential rate <50% (P = 0.004), low pre-CRT T (P = 0.003) and N (P = 0.011) stage, a low post-CRT T (P < 0.001) and N (P = 0.013) stage, a small pretreatment tumor area, and a large difference in tumor area before and after neoadjuvant CRT (P < 0.001) were predictors of a pCR. Multivariate analyses revealed that the difference in tumor area before and after neoadjuvant CRT was the only independent predictor of a pCR (OR, 0.996; 95% CI, 0.993–0.998; P < 0.001).
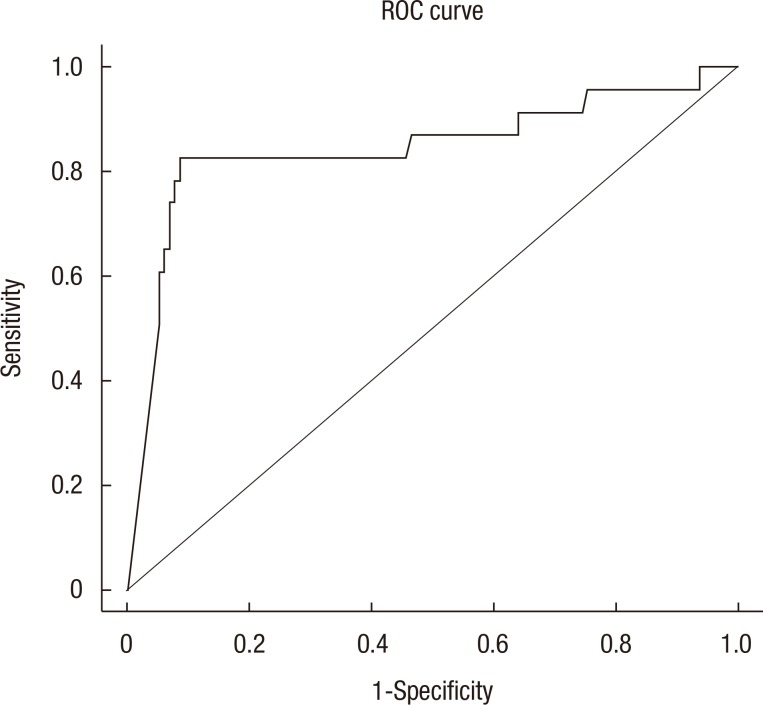
Receiver operating characteristic (ROC) curves of the difference in tumor area to predict pathologically complete response.
DISCUSSION
This study investigated predictors of a pCR by investigating the patients' characteristics, pre-CRT colonoscopic findings, pre- and post-CRT radiological findings and carcinoembryonic antigen levels, and tumor differentiation in locally advanced rectal cancer. The pCR rate in this study was 16.8%, which is similar to the rates found in other randomized phase III trials [202122].
Park et al. [23] investigated the predictors of a pCR in 249 patients with rectal cancer by using a digital rectal exam and colonoscopic findings and found a pCR rate of 12.9%. Multivariate analyses showed that the pre-CRT movability (P = 0.024), post-CRT size (P = 0.018), post-CRT morphology (P = 0.023), and gross changes (P = 0.009) were independent predictors of a pCR. They used colonoscopy to evaluate the pre-CRT circumferential rate, tumor morphology (protruding, flat, or depressed), and the presence of melanosis coli, and only the size-related circumferential rate had a significant relationship with pCR in the univariate analyses.
In a study of 297 rectal cancer patients, Garland et al. [24] reported that a decreased tumor size (P = 0.036) and pretreatment clinical N stage (P = 0.048) were predictors of a pCR. De Felice et al. [25] showed that a pretreatment tumor dimension less than 5 cm was predictive of a pCR; they estimated tumor size by using CT and MRI and determined the tumor area (tumor length × maximum tumor depth). We also showed that the pretreatment tumor area, pre- and post-CRT T and N stages, and difference in tumor area before and after neoadjuvant CRT had significant relationships with pCR in the univariate analyses. In the multivariate analyses, however, the difference in tumor area before and after neoadjuvant CRT was the only predictor of a pCR.
Studies have reported differences in the role of the clinical N stage as a predictor of pCR. As mentioned above, Garland et al. [24] found that the pretreatment clinical N stage was predictive of a pCR while other studies found that it was not [23252627]. This may be because, despite the improvement in imaging modalities, the accuracy of lymph-node assessment is still poor. In a systemic review and meta-analysis of 21 studies, Al-Sukhni et al. [28] suggested that MRI had poor accuracy in lymph-node assessment and reported a 77% sensitivity and a 71% specificity in terms of lymph-node positivity.
Some recent studies have reported a relationship between k-ras mutation and the response to neoadjuvant CRT. Chow et al. [29] showed that k-ras mutation was related to a low pCR rate in patients with locally advanced rectal cancer. Conversely, in a systemic review and meta-analysis, Clancy et al. [30] reported that k-ras mutation did not affect tumor down-staging or cancer-specific survival.
Our study was limited in that it was both a single-center study with a small sample size and a retrospective study based on a chart review. More patients received neoadjuvant CRT for rectal cancer in our center during the study period, but we excluded those who had not undergone post-CRT imaging studies or had refused surgery, thereby leaving 137 patients eligible for this study. A few studies have reported that the longer the interval between preoperative CRT and surgery is, the higher the rate of pCR is [3132]. However, in our center, surgery is routinely preformed 6 weeks after preoperative CRT; therefore, we could not include the time interval as a variable. Moreover, because some patients refused posttreatment colonoscopy, we could not investigate the colonoscopic findings post-CRT.
In conclusion, we found that the difference in tumor area before and after neoadjuvant CRT, as determined radiologically, may be an important predictor of a pCR. Future studies of molecular markers and this clinical measurement may help surgeons select for surgery patients who have received neoadjuvant CRT for the treatment of locally advanced rectal cancer.
ACKNOWLEDGMENTS
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government's Ministry of Science, ICT and Future Planning (MSIP) (No. 2017R1A2B4007725).
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.