Transanal Minimally-Invasive Surgery for Treating Patients With Regressed Rectal Cancer After Preoperative Chemoradiotherapy
Article information
Abstract
Purpose
Although the standard treatment for patients with locally advanced rectal cancer managed by preoperative chemoradiotherapy (CRT) is a radical resection, local excisions are used in highly-selective cases. Recently, transanal minimally-invasive surgery (TAMIS) has emerged as a feasible technique for local excision of midrectal lesions. We assess the feasibility of using TAMIS to treat patients with locally advanced rectal cancer who showed good response to CRT.
Methods
From October 2010 to June 2013, 35 consecutive patients with rectal cancer managed by using preoperative CRT underwent TAMIS. After a single-incision laparoscopic surgery port had been introduced into the anal canal, a full-thickness local excision with conventional laparoscopic instruments was performed. We retrospectively reviewed a prospectively collected database of these cases.
Results
Of the 35 patients analyzed, 18 showed pathologic complete responses and 17 had residual lesions (2 ypTis, 4 ypT1, 9 ypT2, and 2 ypT3); 34 (97.1%) showed clear deep, lateral margins. The median distance of lesions from the anal verge was 5 cm. All procedures were completed laparoscopically, and the median operating time was 84 minutes. No intraoperative events or morbidities were seen in any of the patients, except one with wound dehiscence, who was treated conservatively. The median postoperative hospital stay and follow-up period were 4 days and 36 months, respectively. During the study period, no patients died, but 5 (14.3%) experienced recurrence, including one recurrence at the TAMIS site.
Conclusion
TAMIS seems to be a feasible, safe modality for treating patients with locally advanced rectal cancer who show good response to preoperative CRT.
INTRODUCTION
Although the standard treatment for patients with locally advanced rectal cancer following preoperative chemoradiotherapy (CRT) is a transabdominal resection [1], local excisions are considered as an alternative treatment option in highly selective cases. The results of a recent meta-analysis including 10 retrospective, 1 single-arm prospective, and 1 randomized series showed that local excision may be appropriate for selected patients who show good clinical response after CRT [2].
The usual indication for preoperative CRT is a locally advanced (clinical T3/T4 or node positive) rectal adenocarcinoma located within 10 cm from the anal verge. For very low-lying rectal tumors located around the anal verge, a conventional transanal excision (TAE) can be performed easily and safely. However, for tumors located in the midrectum, conventional TAE is very difficult and does not guarantee a sound oncologic excision. Therefore, transanal endoscopic microsurgery (TEM) is currently the only method for the local excision of tumors located in this area. However, TEM has not been universally adopted by colorectal surgeons, partly because of the steep learning curve and the high cost of specialized instrumentation required for the procedure [34].
Recently, transanal minimally-invasive surgery (TAMIS), an innovative technique that combines single-port access with the principles of TAE, was reported as a feasible alternative to TEM [5]. We have also reported the feasibility and safety of this technique for resection of midrectal lesions [6]. However, previous reports included only benign cases or early rectal cancer; therefore, the feasibility and safety of TAMIS for treating patients with rectal cancer who have undergone preoperative CRT remains to be determined. Long-course CRT administrated prior to surgical resection can cause inflammation and fibrosis in the radiated rectum, which may impact the feasibility of the surgical procedure and postoperative outcome. The aim of this study is to evaluate the feasibility and safety of performing TAMIS in patients with rectal cancer who had undergone preoperative CRT.
METHODS
Between October 2010 and June 2013, 776 consecutive patients with a primary rectal adenocarcinoma underwent preoperative CRT followed by surgery at Asan Medical Center, Seoul, Korea. All patients had the following characteristics: (1) tumors located in the middle or distal rectum (within 10 cm of the anal verge); (2) locally advanced disease (clinically T2/T3 or N+), as determined by pelvic magnetic resonance imaging with transrectal ultrasonography; (3) no previous or concurrent malignancy; and (4) no evidence of distant metastasis on pretreatment workup. Preoperative radiotherapy was delivered to the entire pelvis at a dose of 45 Gy in 25 fractions, followed by a boost to the primary tumor of 5.4 Gy in 3 fractions over 5.5 weeks. The details of the radiotherapy protocol have been previously reported [7]. Concurrent chemotherapy usually consisted of (1) 5-fluorouracil and leucovorin, delivered as 2 cycles of bolus intravenous 5-fluorouracil (375 mg/m2/day) and leucovorin (20 mg/m2/day) for 3 days each during the first and the fifth weeks of radiotherapy (29 patients) or (2) oral capecitabine (825 mg/m2) twice daily during radiotherapy without weekend breaks (4 patients). One patient received oral capecitabine (825 mg/m2) twice daily for 4 weeks with a weekend break with 2 cycles of bolus intravenous oxaliplatin (85 mg/m2) in the first and the third weeks of radiotherapy.
The degree of response to preoperative CRT was assessed by using pelvic magnetic resonance imaging, transrectal ultrasonography, sigmoidoscopy, carcinoembryonic antigen (CEA) levels, and digital rectal examination 1 week before surgical scheduling. Possible indications for local excision were a tumor showing a small whitish scar and/or shallow ulcer on sigmoidoscopy, no fixed lesion on digital rectal examination, and no possible metastatic lymph node on pelvic resonance imaging and transrectal ultrasonography. Of the 776 patients with a primary rectal adenocarcinoma, 63 (8.1%) underwent local excision. Of these 63, 35 consecutive patients underwent TAMIS excision by the same surgeon (SBL). After a single-incision laparoscopic surgery port had beens introduced into the anal canal, a full-thickness local excision with conventional laparoscopic instruments was made. The median interval between CRT and TAMIS surgery was 57 days (range, 47–89 days). All procedures were performed after obtaining informed consent from the patients. The details of TAMIS surgery have been previously reported [6].
Response to CRT was evaluated in the excised specimen by using the tumor regression grade (TRG) scale [8]. All patients received postoperative care according to a protocol and underwent a standardized postoperative follow-up consisting of a physical examination, including digital rectal examination, complete blood count, liver function test, serum CEA analysis, and chest radiography, every three months for the first 2 postoperative years and every 6 months thereafter; abdominal and pelvic computed tomography every 6 months; and chest computed tomography every year. Sigmoidoscopy was performed every 3 months for the first 2 years; then, colonovideoscopy was performed annually. Continence issues and any subjective symptoms were evaluated at 3 months by history taking. Clinicopathological findings, surgical outcomes, and postoperative outcomes were evaluated.
RESULTS
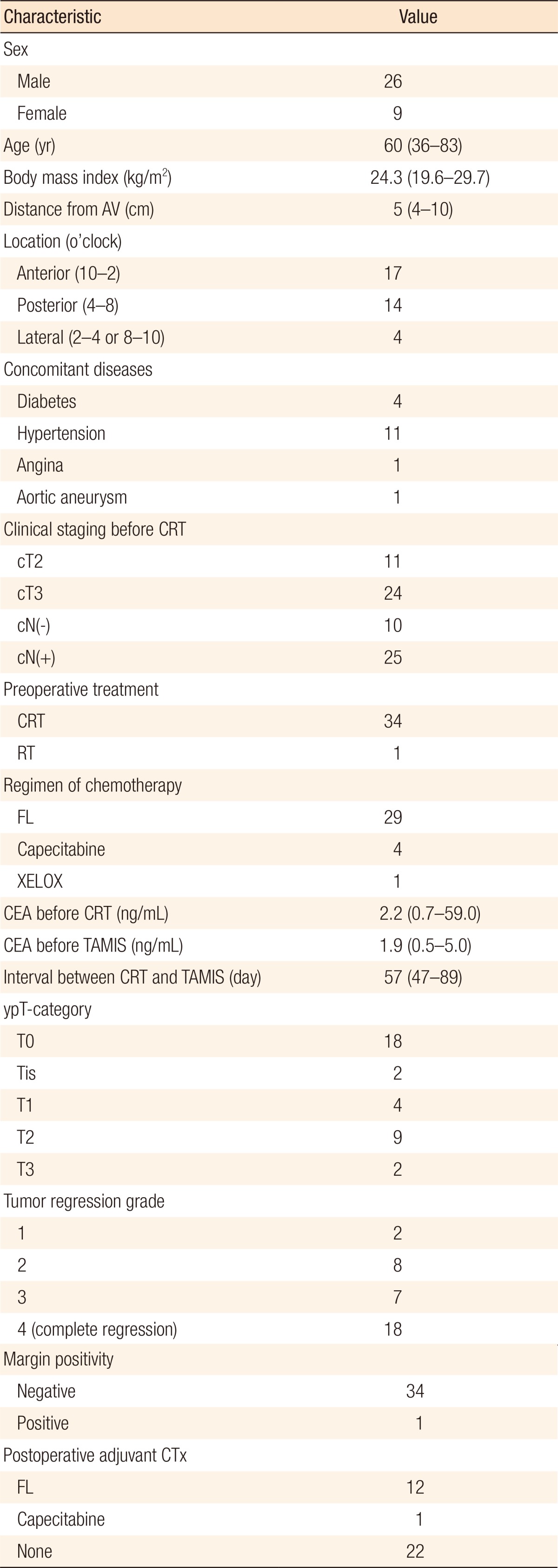
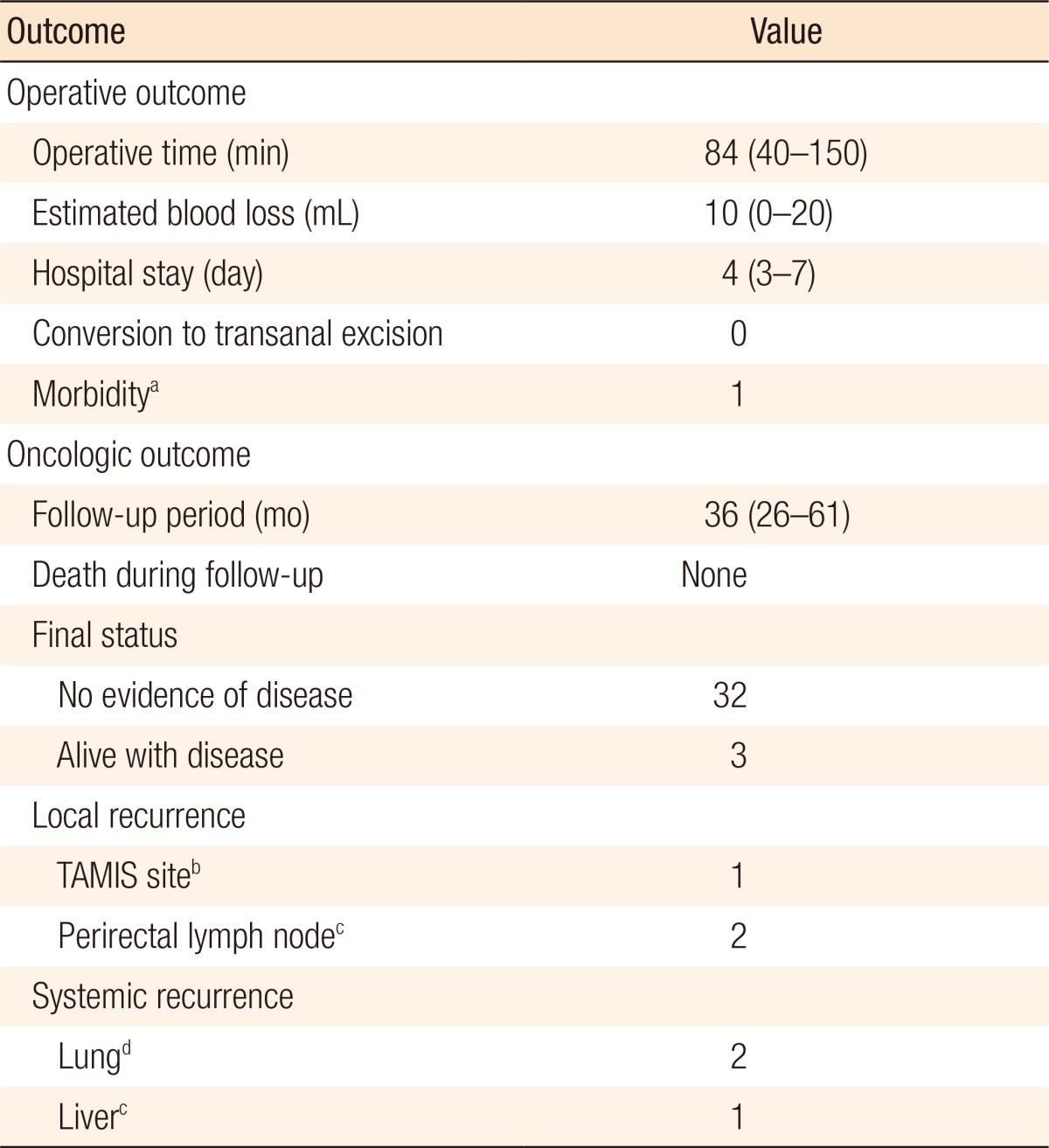
The 35 patients who underwent TAMIS excision were aged 36–83 years; their clinicopathologic characteristics are presented in Table 1. Of these 35 patients, 18 showed a pathologic complete response (TRG 4); 17 had residual lesions, with the pathologic T stages after surgery being 2 ypTis, 4 ypT1, 9 ypT2, and 2 ypT3. Thirty-four patients (97.1%) showed clear, deep, lateral margins. Almost one-third of the patients (11 of 35, 31.4%) were finally diagnosed as ypT2 or ypT3, and a patient showed a positive margin; however, patients wanted organ preservation treatment and refused to undergo surgery. The median distance of the lesions from the anal verge was 5 cm (range, 4–9 cm). All procedures were completed laparoscopically without conversion to a conventional transanal approach. The operative and the postoperative oncologic outcomes are presented in Table 2. The median operating time was 84 minutes (range: 40–150 minutes), and the median estimated blood loss was 10 mL (range, 0–20 mL), with no patient requiring intraoperative transfusions. No mortalities or intraoperative events occurred. The median postoperative hospital stay was 4 days (range, 3–7 days). Follow-up sigmoidoscopy 3 months after the procedure showed an intact suture line in 34 patients while 1 patient had developed wound dehiscence with anal pain which was diagnosed at outpatient care (3 weeks after surgery) and treated conservatively. Neither fecal incontinence nor other anorectal dysfunction was observed, and 2 patients complained of mild anal pain not requiring medication. The median follow-up period was 36 months (range, 26–61 months), and all patients were followed completely.
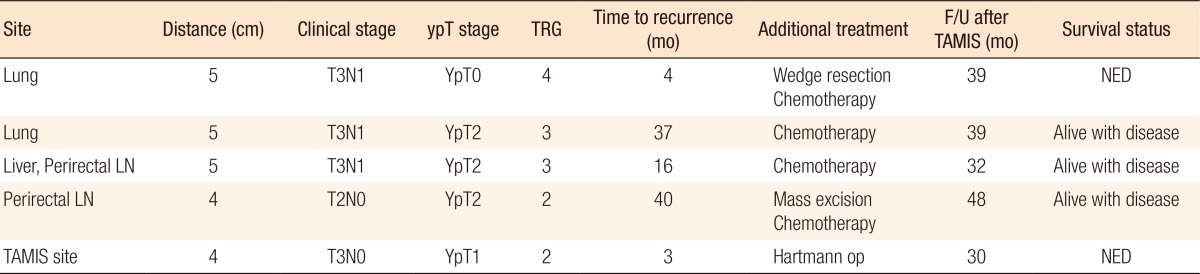
During the follow-up period, no patient died, but 5 patients (14.3%) experienced recurrence. The clinical data for those 5 patients are presented in Table 3. Two patients had local recurrences (1 at the TAMIS excision site and 1 at the perirectal lymph node); 1 of the 2 remains alive and free of disease after salvage op, and the other underwent excision and is managed with chemotherapy. Two patients developed systemic metastases (both at the lung); 1 remains alive and free of disease after a wedge resection and chemotherapy, and the other is being managed with chemotherapy. The remaining patient had both local recurrence and systemic metastases (at the perirectal lymph node and the liver) and received chemotherapy.
DISCUSSION
Currently, 3 treatment options are available after preoperative CRT in patients who show a clinically complete response: a conventional total mesorectal excision (TME), which is considered as the standard treatment, the watch and wait policy, and local excision. Among these options, local excision is considered as an attractive option in highly-selective patients showing a major clinical response after CRT. Local excision results in a better quality of life with low morbidity and is stoma-free compared to conventional TME, and some prospective studies suggest that CRT before local excision reduces recurrence to a level comparable to that of TME [91011]. The advantage of local excision, compared with watch and wait, is that an apparently good clinical response can be confirmed histopathologically on a complete specimen.
A major concern in performing local excision, besides the oncologic feasibility, is the technical safety related to the location of the tumor. Usually tumors requiring preoperative CRT are located in the mid to low rectum. For low-lying rectal tumors situated around the anal verge, conventional TAE can be adequate and safe. In contrast, for midrectal cancers, obtaining a good surgical view and an adequate oncologic margin with conventional TAE is very difficult because of the narrow nature of the anal canal. Hence, other local minimally-invasive techniques, such as TEM, are needed. Recent studies evaluating the efficacy of local excision in such patients have used the TEM technique as an approach of choice [1012]. However, the widespread adoption of TEM faces many barriers, such as the need for specialized instruments, higher associated costs, and a steep learning curve.
TAMIS, a relatively innovative modality, facilitates excision of lesions not otherwise amenable to standard TAE, thereby extending the utility of TAE for lesions of the middle and even upper rectum. The feasibility and safety of TAMIS for local excision of a rectal lesion was reported [5613], and the range of its application has widened beyond local excision to robotic TAMIS [14] and retrograde TAMIS protectomy [15]. Except for TAMIS-proctectomy, the TAMIS approach has almost always been used for local excision of early rectal cancers, benign rectal polyps, and neuroendocrine tumors. In a previous report [6], we suggested the possibility of using TAMIS excision for patients with locally advanced rectal cancer who respond well to CRT to confirm mural sterilization and remove minute foci of the residual tumor. However, definite conclusions could not be made due to the small number of cases (n = 8) and the short follow-up period (<3 months) [6]. To the best of our knowledge, our present study is the first report to show the feasibility and safety of using TAMIS for local excision in patients with locally advanced rectal cancers who show a good response to preoperative CRT. Operative outcomes, such as operative time, estimated blood loss, hospital stay, and morbidity, were found to be comparable to those of conventional TAE. Only one patient experienced wound dehiscence, but could be managed conservatively. Transfusion or conversion to conventional TAE was not needed in any of the patients.
Local excision after CRT using the TEM platform has been previously described. The use of TEM in the setting of neoadjuvant CRT should be considered with care because postoperative wound separation, significant postoperative pain, and the need for hospital readmission are quite significant [16]. In this study, wound dehiscence was 61%, and a possible explanation of these high incidence of wound separation could be the sewing of previously irradiated tissues. Compared to the high rate (60% to 72%) of wound complications using TEM [121617], the rate of wound dehiscence using TAMIS in our present study was extremely low (1 of 35, 2.9%). The better wound-healing outcome may be associated with the use of a soft single-incision laparoscopy port instead of a rigid proctoscope to obtain the operative field. Furthermore, in our present study, all patients showed a good continence level and had no specific complaints related to bowel movements requiring medication during outpatient follow-up. No comparative trials exist that have compared perioperative outcomes between TEM and TAMIS for this patient group.
Our present investigation also highlighted the oncologic safety of the TAMIS platform for local excision for patients with locally advanced rectal cancer who responds well to CRT. During the follow-up period (median, 36 months; range, 16–61 months), in our cohort of patients, none were lost to cancer-related death, and five (5 of 35, 14.3%) experienced recurrences. Two had local recurrences (1 at the TAMIS excision site, and 1 at the perirectal lymph node), 2 had systemic metastases (both at the lungs), and 1 developed local and systemic recurrence (at the perirectal lymph node and liver). The local recurrence sites were detected using a close surveillance program (sigmoidoscopy every 3 months and computed tomography every 6 months) and were managed immediately (salvage proctectomy and chemotherapy).
The present study has some limitations of note. First, this was a single-institute study with a small cohort. Although standard laparoscopic instruments were used, appropriate laparoscopic skills are needed to ensure the procedure is performed safely and completely. Furthermore, this study was retrospective and thus susceptible to the inherent limitations associated with such a design. We analyzed a relatively-homogenous patient group, but their pathologic statuses were heterogeneous, thus limiting the interpretation of our oncologic results. In conclusion, TAMIS shows promise as a feasible and safe approach modality for local excision in patients with locally advanced rectal cancer who show a good response to preoperative CRT and should be considered as an approach of choice for local excision in such patients.
ACKNOWLEDGMENTS
The authors thank all of the anesthesiologists and surgical nurses at Asan Medical Center for their assistance in the development of the TAMIS technique.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.