Concurrent Large Cell Neuroendocrine Carcinoma and Adenocarcinoma of the Ascending Colon: A Case Report
Article information
Abstract
Large cell neuroendocrine carcinomas of the colon are rare and represent only a small percentage of all colonic endocrine tumors. Here, we report a case of a colonic large cell neuroendocrine carcinomas concurrent with a colonic adenocarcinoma. A 70-year-old man presented with acute abdominal pain. A spiral computed tomography scan of the abdomen revealed eccentric wall thickening on the ascending colon. An explorative laparotomy and a right hemicolectomy were performed. Grossly, two separated masses were observed in the proximal ascending colon. One was a 7.4 × 5.1 cm ulcerative fungating lesion, and the other was a 2.8 × 1.9 cm polypoid lesion. Microscopically, the ulcerative fungating lesion showed a well-differentiated neuroendocrine morphology with necrosis and increased mitosis. Most of the tumor cells had large, vesicular nuclei with eosinophilic nucleoli, variable amounts of eosinophilic cytoplasm, and immunoreactivity for chromogranin A and synaptophysin. The polypoid lesion was a well-differentiated adenocarcinoma that had invaded the submucosa. We diagnosed these lesions as a concurrent large cell neuroendocrine carcinoma and an adenocarcinoma of the ascending colon.
INTRODUCTION
The current World Health Organization histologic classification of colorectal endocrine tumors includes well-differentiated neuroendocrine neoplasms, well-differentiated neuroendocrine carcinomas, and poorly-differentiated neuroendocrine carcinomas [1]. Neuroendocrine carcinomas of the colon and rectum are rare, representing 0.1-3.9% of colorectal cancers [2]. Furthermore, large cell neuroendocrine carcinomas (LCNECs) of the colon are extremely rare, representing 0.25% of colorectal cancers [2]. In the colon and the rectum, most concurrent tumors are those that are composed of an adenocarcinoma and a well-differentiated neuroendocrine neoplasm (carcinoid) or an adenoma and a carcinoid. A combination of a colorectal LCNEC and a colorectal adenocarcinoma is extremely rare, and only three cases have been reported [3-5]. Here, we present a rare case of a colonic concurrent tumor involving a LCNEC and an adenocarcinoma.
CASE REPORT
A 70-year-old man presented with acute abdominal pain lasting several hours. The past history was unremarkable. Physical examination revealed direct tenderness and rebound tenderness on the entire abdomen. The complete blood cell count showed an increased leukocytes (16,900/µL). Contrast-enhanced computed tomography (CT) scans revealed eccentric wall thickening with mucosal enhancement of the proximal ascending colon (Fig. 1A). There were multiple air bubbles at the pericolic area, suggestive of bowel perforation. The levels of carcinoembryonic antigen and carbohydrate antigen 19-9 were within normal limits. The patient underwent an emergency explorative laparotomy and a right hemicolectomy.

(A) Contrast-enhanced computed tomography scan reveals eccentric wall thickening with mucosal enhancement of the proximal ascending colon (arrow). (B) Gross finding of the colonic lesion shows two separated masses. One is an ulcerative fungating mass (black arrows), and the other is a polypoid mass (arrowheads). There are several sessile polypoid lesions in the adjacent mucosa (white arrows).
Macroscopically, the cecal wall showed necrotic change with perforation through which fecal materials had leaked. The main colonic lesion was located in the middle of ascending colon, causing near-complete obstruction of the lumen, and was composed of two separated masses. One was a 7.4 × 5.1 cm fungating mass with ulceration in the center (Fig. 1B, black arrows). The cut surface revealed a white-gray color, solid, poorly demarcated mass with focal necrosis. The other was a 2.8 × 1.9 cm polypoid mass (Fig. 1B, arrowheads), and it was located about 1 cm distally apart from the ulcerative fungating mass. Several sessile polypoid lesions measuring less than 0.5 cm were also detected (Fig. 1B, white arrows). In addition to the right colon specimen, 29 resected regional lymph nodes were submitted.
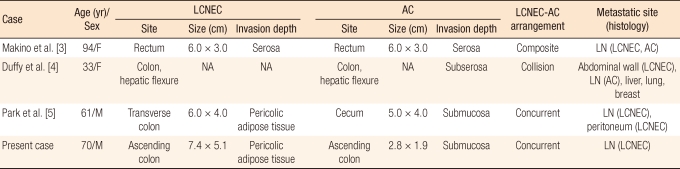
Microscopically, the ulcerative fungating mass was separated abruptly from the normal colonic mucosa (Fig. 2A). It involved from the mucosa to the pericolic adipose tissue and showed well-differentiated neuroendocrine morphology, such as nests, trabeculae, acini and rosettes (Fig. 2B). A focal area of tumor cells with an individually infiltrative or an Indian file-like arrangement was noted (Fig. 2C). The majority of the tumor cells had large, vesicular nuclei with prominent eosinophilic nucleoli and variable amounts of eosinophilic cytoplasm (Fig. 2D). There were commedo-type necrosis, apoptotic bodies and high mitotic figures (10/10 HPF). The polypoid mass was a well-differentiated adenocarcinoma that was mainly composed of irregularly branching tubules and that had invaded the submucosa (Fig. 2E). Adjacent sessile polypoid lesions were hyperplastic polyps. Regional lymph node metastasis was detected in 11 out of 29 lymph nodes.
(A) Ulcerative fungating mass is separated abruptly from the normal colonic mucosa and shows a trabecular growth pattern (H&E, × 40). (B) It also shows nests and rosettes with comedo-type necrosis (H&E, × 100). (C) Some tumor cells reveal an individually infiltrative or an Indian file-like arrangement (H&E, × 200). (D) Tumor cells are arranged in rosettes and had large, vesicular nuclei with prominent eosinophilic nucleoli and eosinophilic cytoplasm (H&E, × 400). (E) The polypoid mass is composed of irregularly branching tubules and invades the submucosa (H&E, × 40). (F) The histologic features of the metastatic lymph nodes are similar to those of colonic large cell neuroendocrine carcinoma (H&E, × 20).
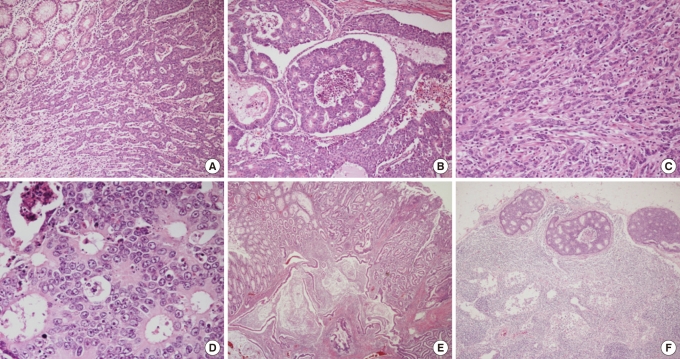
The histologic features were similar to those of colonic LCNEC (Fig. 2F). The tumor cells of the ulcerative fungating mass had perinuclear, dot-like or cytoplasmic immunoreactivity for chromogranin A (Fig. 3A) and synaptophysin. On the other hand, the tumor cells of the polypoid mass had no immunoreactivity for chromogranin A (Fig. 3B) and synaptophysin. Both the tumor cells of the ulcerative fungating mass and of the polypoid mass had immunoreactivity for CK20 (Fig. 3C, D). Paraffin-embedded tissue samples for immunohistochemistry were provided by Chonbuk National University Hospital, a member of the National Biobank of Korea, which is supported by the Ministry of Health, Welfare and Family Affairs. We diagnosed these lesions as a concurrent LCNEC and adenocarcinoma of the ascending colon. Though we recommended adjuvant chemotherapy with the FOLFOX (Lecovorin, 5-FU, Exolatin) regimen after a right hemicolectomy, the patient rejected our suggestion.
(A) The tumor cells of the ulcerative fungating mass have perinuclear, dot-like or cytoplasmic immunoreactivity for chromogranin A (× 200). (B) The tumor cells of the polypoid mass have no immunoreactivity for chromogranin A (× 200). Note the immunoreactive endocrine cells in the glands as an internal positive control. (C) The tumor cells of the ulcerative fungating mass have strong cytoplasmic immunoreactivity for CK20 (× 100). (D) The tumor cells of the polypoid mass have cytoplasmic immunoreactivity for CK20 (× 100).
DISCUSSION
Neuroendocrine tumors are rare neoplasms originating from the neuroendocrine system. They are classified into three groups: 1) well-differentiated neuroendocrine tumors showing benign behavior or uncertain malignant potential (carcinoid), 2) well-differentiated neuroendocrine carcinomas, which are characterized by low-grade malignancy (malignant carcinoid) and 3) poorly differentiated, usually small cell, neuroendocrine carcinomas of high-grade malignancy [1]. Most neuroendocrine tumors of the colon and the rectum are carcinoids. Well-differentiated neuroendocrine carcinomas are aggressive lesions and represent poorly-differentiated forms of carcinoid tumors with increased mitotic activity and absent or limited extent of necrosis.
The LCNEC is a newly defined high-grade malignant tumor entity of a non-small-cell-type neuroendocrine tumor, which is well established in pulmonary neuroendocrine tumors. A colonic LCNEC is a rare malignant neoplasm composed of large cells having organoid, nesting, trabecular, rosette-like and palisading patterns that suggest neuroendocrine differentiation. Tumor cells are generally in polygonal or oval shapes and show hyperchromatic or vesicular nuclei with prominent nucleoli and slightly eosinophilic cytoplasm with ill-defined cellular boundaries [1]. In contrast to small-cell carcinomas, the cytoplasm is more abundant, nuclei are more vesicular, and nucleoli are prominent.
Among colonic neuroendocrine tumors, 48% of the cases arise in the cecum, 16% in the ascending colon, 6% in the transverse colon, 11% in the descending colon, and 13% in the sigmoid colon. Bernick et al. [2] reported that 0.6% of patients with colorectal cancer were affected by neuroendocrine carcinomas and 0.2% by LCNECs. Most LCNECs located in the rectum and the cecum present in an advanced stage.
Colorectal LCNECs can be confirmed by immunohistochemistry and electron microscopy as long as a microscopic neuroendocrine morphology is present. However, the diagnoses of colorectal LCNECs are often too difficult because colorectal carcinomas frequently exhibit neuroendocrine differentiation in 20-50% of cases, and neuroendocrine differentiation is more frequent in midgut-derived carcinomas than in those of hindgut origin [6, 7]. Therefore, the most important issue is to distinguish colorectal LCNECs from other colorectal carcinomas with neuroendocrine differentiation. In the present case, a definite neuroendocrine morphology was recognized even though a focal area of vague, undifferentiated morphology existed. We believe that the recognition of an organoid architecture is a good initial indication of the presence of a colorectal LCNEC and that a subsequent immunohistochemical stain may possibly contribute to a differential diagnosis. In immunohistochemical staining, LCNECs express cytokeratins mimicking poorly-differentiated adenocarcinomas. However, in contrast to poorly-differentiated adenocarcinomas, LCNECs express neuroendocrine markers.
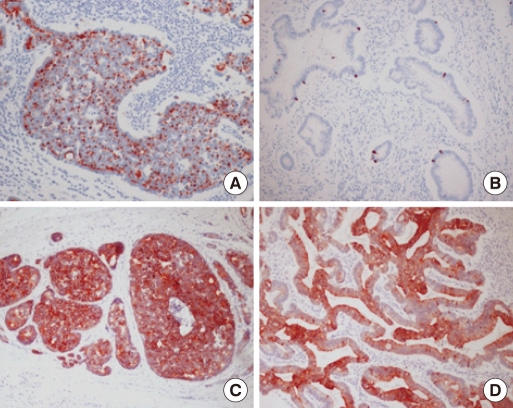
Colorectal neruendocrine tumors are associated with secondary primary tumors, including both adenocarcinomas and other carcinoid tumors, in other sites, particularly in the GI tract. The overall incidence of secondary primary tumors ranges from 3% to 15% [8, 9]. LCNECs may be associated with an adjacent adenoma or a conventional adenocarcinoma. A combination of a colorectal LCNEC and a colorectal adenocarcinoma has only been reported in three cases (Table 1) [3-5]. Two of them involved a mixed tumor of either composite or collision type whereas the other involved a synchronous tumor as in the present case.
The precise pathogenesis of secondary primary tumors in patients with established neuroendocrine tumors remains unclear. One proposed hypotheses is the field effect, stimulated growth of neuroendocrine tumor cells and secondary primary tumor cells caused by common carcinogens. Another proposed hypothesis is the stem cell theory, which relates the potency of various differentiations to several tumor types [10]. However, these hypotheses seem unlikely because a significant percentage of second primary malignancies occur in the genitourinary tract or even at more distant sites. The other proposed hypothesis is a common genetic alteration that predisposes an individual to the development of neuroendocrine tumors and second primary tumors. In addition, Kato et al. [11] reported a CK20 positive LCNEC of the colon and suggested a possible link between a colorectal neuroendocrine carcinoma and adenocarcinoma. This report is consistent with the immunohistochemical findings in our case, where the patient had an advanced stage of LCNEC and a simultaneous early-stage adenocarcinoma in the ascending colon. However, more work is required to clarify the pathogenesis of a concurrent colorectal LCNEC and adenocarcinoma.
Although tumor size and microinvasion are major prognostic features in other GI neuroendocrine tumors, these features tend to be less useful in assessing the prognosis of colonic neuroendocrine tumors because most of these lesions exceed 2 cm in size and involve the muscularis propria at the time of presentation [9]. Only 16.6% of colonic neuroendocrine tumors less than 2 cm in size metastasize whereas 74% of those more than 2 cm in size metastasize to the lymph nodes, liver, mesentery, peritoneum, pancreas, ureters, ovaries, and omentum [8]. Therefore, colonic neuroendocrine tumors can be treated by local excision if they are found when they are small; however, larger tumors should be treated aggressively with a standard colonic resection and lymph node dissection. Mitotic rate, overall tumor grade, and histologic pattern all influence the survival for patients with colonic neuroendocrine tumors [9].
Colorectal LCNECs should be taken as a distinct histopathologic entity because they have a significantly worse prognosis than conventional adenocarcinomas. This paper presented a rare example of two separated colonic tumors in a unique combination, namely, a concurrent LCNEC and adenocarcinoma of the ascending colon.
Notes
No potential conflict of interest relevant to this article was reported.