Effectiveness of Adjuvant Chemotherapy with 5-FU/Leucovorin and Prognosis in Stage II Colon Cancer
Article information
Abstract
Purpose
The aims of this study were to investigate the survival results and the prognostic factors of adjuvant chemotherapy in stage II colon cancer in the sparsity of Korean data.
Methods
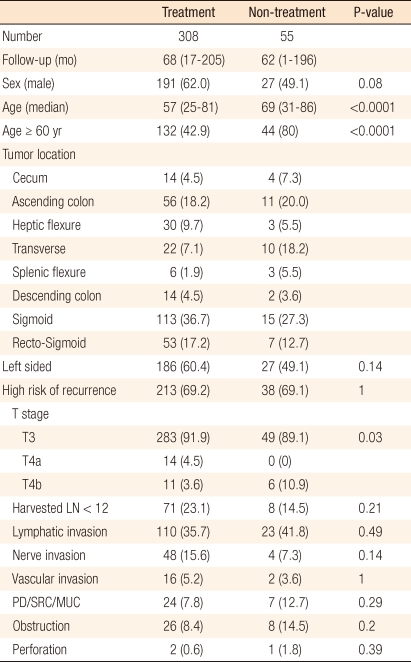
From 1993 to 2006, 363 curatively resected pathologic stage II colon cancer patients were enrolled. Six cycles of adjuvant chemotherapy was performed: intravenous bolus 5-fluorouracil (5-FU) 500 mg/m2 with leucovorin 20 mg/m2 for 2 hours daily for 5 days, followed by a 3-week resting period (n = 308). Fifty-five patients received only curative surgery. A high risk of recurrence was defined as the presence of one or more of the following factors: T4 tumor, lympho-vascular invasion, perineural invasion, perforation, obstruction, retrieved lymph node < 12, and poorly differention. The median follow-up period was 68 months (1 to 205 months).
Results
The five-year overall survival (OS) rate was 90.1%, and the five-year disease-free survival (DFS) rate was 84.7%. Among high-risk patients, the OS and the DFS rates of the treatment group were significantly higher than those of the non-treatment group (OS: 90.6% vs. 69.1%, P < 0.0001; DFS: 85.9% vs. 54.1%, P < 0.0001). Among low-risk patients, the survival results of the treatment group were also significantly superior (OS: 97.7% vs. 88.2%, P < 0.0001; DFS: 93.0% vs. 80.0%, P = 0.001). In the multivariate analysis, adjuvant chemotherapy was a significantly favorable prognostic factor for overall survival (hazard ratio, 0.41; 95% confidence interval, 0.22 to 0.75; P = 0.004).
Conclusion
In our population, adjuvant chemotherapy showed superior survival to curative surgery alone and significantly reduced the risk of death. A nationwide multicenter randomized trial is needed.
INTRODUCTION
Colorectal cancer is one of major cause of death in the west. However, according to 2008 statistics in Korea, the annual increase in the number of colon cancer cases has reached 5.3% and colon cancer is the third most common type of cancer [1].
Although the most important factor for the treatment of colon cancer is a curative resection, adjuvant chemotherapy following such curative resection has reduced the recurrence and mortality in locally advanced cases [2]. However, the benefit of adjuvant chemotherapy has been demonstrated usually for patients with lymph-node metastasis of stage III colon cancer. The benefits for patients wirh stage II colon cancer are still controversial. The IMPACT B2 study showed statistically insignificant effects of about 2% in the treatment group when compared to the non-treatment group in stage II colon cancer, but the QUASAR study performed in 2007 showed a small, but statistically significant, difference of 3.6% [3, 4].
As to the effectiveness of adjuvant chemotherapy in patients with stage II colon cancer, which has rarely been reported in Korea, the authors identified the effects of adjuvant chemotherapy using intravenous 5-fluorouracil (5-FU)/leucovorin (LV) in patients with stage II colon cancer who underwent a curative resection by comparing the survival rate with that of the non-treatment group. In addition, the prognostic effects of adjuvant chemotherapy for stage II colon cancer were investigated using a multivariate analysis.
METHODS
Patients
From 1993 to 2006, 363 colon adenocarcinoma patients who underwent a curative resection and were diagnosed as having stage II colon cancer at the Korea Cancer Center Hospital were retrospectively analyzed. The inclusion criteria was following. The distal part of the tumor was located 15 cm above the anal verge. Histologically, the tumor had invaded beyond the muscle layer to the serosa or pericolic fat tissue, and no lymph node metastatis or systemic metastasis was found. Rectal cancer and patients with a history of treatment for other cancer, metachronous colon cancer, recurring colorectal cancer and familial adenomatous polyposis or other hereditary colon cancer syndromes were excluded.
The adjuvant chemotherapy was performed in patients aged over 18 years with no history of receiving chemotherapy or radiation treatment and with proper hematological findings, hepatic and renal function after providing sufficient information on the procedure and obtaining their consents.
Treatment
The adjuvant chemotherapy was initiated at 3-8 weeks postoperative. It was performed 6 times at 4-week intervals. The patient was treated by intravenous infusion of LV, 20 mg/m2, with subsequent intravenous infusion of 5-FU, 500 mg/m2, for 2 hours daily for 1 to 5 days.
Clinicopathological characteristics
Gender, age, T stage in accordance with the Classification Version 7 published by the American Joint Committee on Cancer (AJCC) [5], harvested lymph nodes, the grade of differentiation, the presence of lymphatic or vascular invasion, obstruction, perforation, recurrence and survival were investigated retrospectively. The high-risk group of stage II colon cancer included patients with any of the following: T4 tumors, the presence of tumors with low histologic grade, such as, poorly differentiated carcinomas, and undifferentiated carcinomas; the presence of lymphatic, vascular or neural invasion; the presence of obstruction or perforation; or harvested lymph nodes less than 12. When patients had none of these risk factors, they were categorized as the low-risk group.
Follow-up
Patients were followed at 3-month intervals for 3 years postoperative. During the 4th and the 5th year after surgery, they were observed at 6-month intervals, and after the 5th year, they were observed annually. Physical examination, serum carcinoembryonic antigen (CEA) level, carbohydrate antigen (CA) 19-9 level and chest X-ray were performed every time. Computerized tomography of the thorax and abdomen was performed at 6-month to 1-year intervals. The median follow-up period was 68 months (1 to 205 months).
Statistical analysis
The statistical analysis was performed using SPSS ver. 14.0 (SPSS Inc, Chicago, IL, USA). In the comparison among each variable, the Fisher's exact test was used for nominal variables whereas the independent sample T test or the Mann-Whitney U test was used for continuous variables, depending on the presence of normality. As the method of survival analysis, the Kaplan-Meier's method was used, and a log-rank analysis was performed for inter-group differences. In order to identify the effects of adjuvant chemotherapy on the prognosis, the Cox-proportional hazard model was used for the univariate and the multivariate analyses. From the univariate analysis, variables having significant values were selected; then, the multivariate analysis was performed on them. When the significance level was smaller than 0.05, it was considered as statistically significant.
RESULTS
The median age of all patients was 59 years (range, 25 to 86 years) and the male was predominant gender. The treatment group, which received the intravenously infused 5-FU/LV, included 308 patients (84.8%). The non-treatment group, which received no adjuvant chemotherapy, included 55 patients (15.2%). Among the stage II colon cancer, 251 patients (69.1%) were in the high-risk group, and 112 patients (30.9%) were in the low-risk group.
In the high-risk group, T4 tumors were present in 31 patients (8.6%), T4a in 14 patients (3.9%) and T4b in 17 patients (4.7%). Low histologic grade tumors, such as poorly differentiated carcinomas, undifferentiated carcinomas and mucous carcinoma were present in 31 patients (8.5%), lymphatic invasion in 133 patients (36.6%), vascular invasion in 18 patients (5.0%), neural invasion in 52 patients (14.2%), bowel obstruction in 34 patients (9.4%), and bowel perforation in 3 patients (0.8%). The number of resected lymph nodes was less than 12 in 79 patients (36.6%) (Table 1).
Recurrence occurred in 32 patients. The recurrence rate was higher in the high-risk group (26 patients, 10.4%) than in the low risk group (6 patients, 5.4%), but this difference had no statistical significance (P = 0.16). Among the 23 patients identified with recurrence, hematogeneous metastasis (16 patients, 69.6%) was the most prevalent, followed by local recurrence (4 patients, 17.4%), distant lymph node metastasis (2 patients, 8.7%) and peritoneal metastasis (1 patient, 4.3%) in order. In hematogeneous metastasis, hepatic metastasis was the most frequent (9 patients, 56.3%), followed by pulmonary metastasis (6 patients, 37.5%) and bone metastasis (1 patient, 6.3%).
The median period until recurrence was 23 months (range, 5 to 11 months). Twenty-five patients died due to recurrences. The total number of deaths was 55.
Dependence of survival rate on adjuvant chemotherapy
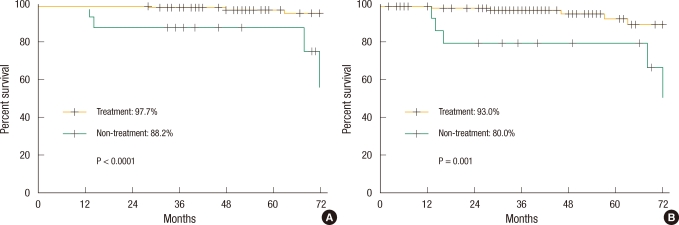
For all 365 patients, the 5-year disease-free survival rate (DFS) and the 5-year overall survival rate (OS) were 84.7% and 90.1%, respectively. The 5-year OS of the treatment group was 92.8%, being significantly higher than the 74.3% for the non-treatment group (P < 0.0001). The 5-year DFS also showed a significant difference, being 87.9% in the treatment group compared to 62.4% in the non-treatment group (P < 0.0001).
In the 251 high-risk patients, the 5-year DFS and OS were 81.9% and 87.4%, respectively. The 5-year DFS and OS of the treatment group were 85.9% and 90.6%, respectively, as compared to 54.1% and 69.1% in the non-treatment group, differences that were statistically significant (P < 0.0001, P < 0.0001) (Fig. 1).
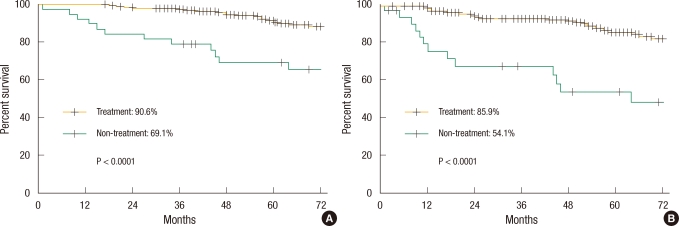
Kaplan-Meier survival curve in high-risk patients: (A) overall survival and (B) disease-free survival.
For the 112 low-risk group patients, the 5-year DFS and the 5-year OS were 91.2% and 96.2%, respectively. The 5-year DFS and the 5 year OS of the treatment group were 93.0% and 97.7%, respectively, which were significant higher survival rates compared to the 80.0% and 88.2% for the non-treatment group (P = 0.001, P < 0.0001) (Fig. 2).
Effects of adjuvant chemotherapy and prognostic factors for patients with stage II colon cancer
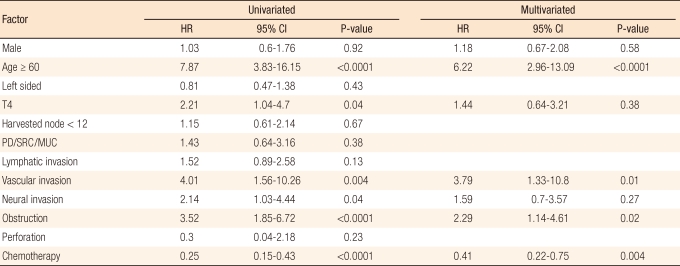
In the univariate analysis, patient age ≥ 60 yrs (hazard ratio [HR], 7.87; 95% confidence interval [CI], 3.83 to 16.15; P < 0.0001), T4 tumor (HR, 2.21; 95% CI, 1.04 to 4.7; P = 0.04), vascular invasion (HR, 4.01; 95% CI, 1.56 to 10.26; P = 0.004), neural invasion (HR, 2.14; 95% CI, 1.03 to 4.44; P = 0.04) and obstruction (HR, 3.52; 95% CI, 1.85 to 6.72; P < 0.0001) were statistically significant factors that affected the overall survival as poor prognostic factors. Adjuvant chemotherapy reduced the risk of death with statistical significance (HR, 0.25; 95% CI, 0.15 to 0.43; P < 0.0001).
In the multivariate analysis, patient age ≥ 60 (adjusted HR, 6.22; 95% CI, 2.96 to 13.09; P < 0.0001), vascular invasion (adjusted HR, 3.79; 95% CI, 1.33 to 10.8; P = 0.01) and obstruction (adjusted HR, 2.29; 95% CI, 1.14 to 4.61; P = 0.02) were independent risk factors affecting the overall survival. In addition, adjuvant chemotherapy was independent favorable prognostic factor; the adjusted HR was 0.41 (95% CI, 0.22 to 0.75; P = 0.004) (Table 2).
DISCUSSION
Colon cancer is one of major cancers resulting in death worldwide. In Korea, it accounts for 12.7% of all cancers incidence, as a westernized dietary has been rapidly spreading, and the number of new cases of colon cancer is showing a stiff increase [1]. Approximately in the past 10 years, the results of colon cancer treatment have improved owing to early diagnosis, prevention and advancement of surgical techniques, but still about 1/3 of patients show recurrence of the disease. The most important factor for survival of patients with colon cancer is the radical surgery, and the survival period of patient is determined based on the postoperative pathological stage of the disease.
Cases clearly showing the benefits of adjuvant chemotherapy are known to involve stage III colon cancer accompanied by lymph node metastasis, with a report on reduction in the risks of death and recurrence by 20-33% from 5-FU mono- or oxaliplatin combined treatment [6, 7], allowing no objections on the effectiveness of adjuvant chemotherapy. However, in reality, still there is no consistent consensus on whether adjuvant chemotherapy is required for patients with stage II colon cancer having no lymph node metastasis and on what kind of chemotherapy regimen should be used. Except QUASAR trial presented in 2007 [4], most studies on adjuvant 5-FU/LV treatment for stage II colon cancer could not identify the statistical superiority of adjuvant treatment over the curative surgery alone [3, 8-10], and such result is because of the difficulty in performing any large-scale clinical study to the extent necessary to verify the superiority of the 5-FU/LV therapy. One study reported that in order to identify the significant difference made by adjuvant chemotherapy in patients with stage II colon cancer in a clinical study, more than 9,000 cases would be required [11]. For these reasons, many studies on adjuvant chemotherapy in patients with stage II colon cancer employed either pooled analysis or meta-analysis using existing studies.
This study aimed to identify the effects of 5-FU/LV adjuvant chemotherapy without oxaliplatin in patients with pathologically proven stage II colon cancer. The results showed statistically significant differences in survival rate for the group on whom adjuvant chemotherapy in both the high- and low-risk subgroup. The results also showed that adjuvant chemotherapy was a significant prognosis factor affecting the overall survival. Although any direct comparison was difficult, when compared to an 83.7% 5-year DFS for patients with stage II colon cancer obtained from the MOSAIC trial using the FOLFOX4 regimen, the 5-year DFS of the our adjuvant chemotherapy group was 87.9%, which was either comparatively higher or similar. For the high-risk group, the 5-year DFS of our adjuvant treatment group was 85.9%, and when compared to the DFS of 82.3% obtained from the MOSAIC trial, the level of survival was similar. For now, it is quite difficult to identify clear reasons for these results. They could be due to differences in the doses of intravenously infused 5-FU (500 mg/m2) at our institution, differences in the sensitivity, which depends on the race and differences in the developmental gene expression of the tumor; nonetheless, above all, it is most likely the result of treatment performed by experienced colon cancer specialists at a single center above all.
According to studies being presented in recent days, the performance of postoperative adjuvant chemotherapy and its regimens for stage II colon cancer are recommended differently depending on whether the patients have the high-risk features for recurrence. In the MOSAIC study, the FOLFOX therapy, which was used for all patients with stage II colon cancer, did not show significant better results compared to monotherapy of 5-FU in terms of overall survival rate or disease-free survival rate. However, in stage II cases with T4 tumors, bowel obstruction, poor differentiation, vascular invasion or with the number of retrieved lymph nodes less than 10, considering them as the high-risk group for recurrence, the FOLFOX therapy showed that survival tended to improve, and the 6-year follow-up observation reported the same result (HR for DFS, 0.76; 95% CI, 0.49 to 1.06) [12]. On the contrary, the benefits of FOLFOX therapy were not identifiable in the group with no risk factors for recurrence. Based on these study results, FOLFOX therapy had been recommended for high-risk patients with stage II cancer, and in 2006, the use of oxaliplatin was approved in high-risk patients with stage II cancer risk in Korea. However, from the NSABP C-07 study conducted under similar design, the benefits of FOLFOX therapy were identified only in stage III colon cancer [13], and also according to a report based on pooled analysis from NSABP C-05, C-06, C-07, and C-08 at the annual meeting of the 2011 American Society of Clinical Oncology (ASCO), an investigation on the treatment result of FOLFOX therapy in 991 patients with stage II colon cancer found only insignificant differences of 2-3% from both the high- and low-risk group, where patients with intestinal perforation or T4 stage of disease and with the number of harvested lymph nodes less than 12 were defined as the high-risk group; the investigation also found that the therapy was seemingly not so effective as to warrant risking further danger such as long-term neurotoxicity [14]. Therefore, even though our study subjects were a group of patients retrospectively recruited by a single center, in consideration of the facts that the 5-FU treatment group had shown better survival result than the FOLFOX treatment group of the MOSAIC study, or that the cancer treatment results of Korea had shown a higher than those of the western countries [15], further studies will be required on how much more beneficial FOLFOX therapy would be as it expresses a far higher frequency of toxic reaction including neurotoxicity, compared to 5-FU/LV therapy in patients with stage II colon cancer and on which patient group should be subjected to such benefits if it would be beneficial at all [16].
In fact, according to the disease stage classification currently in use [5], stage II colon cancer is simply defined as cases in which a tumor with no lymph node metastasis has invaded beyond the proper muscle of the colon. However, such a definition includes a quite diversified wide range of tumors from tumors with no other specific risk factors to tumors that aggressively invade surrounding organs and show findings of a wide range of vascular invasion. To this end, some tumors show prognosis that are consistent with or even worse than that of stage III cancer in terms of recurrence in spite of no lymph node metastasis [17, 18]. Thus, investigations have been implemented on various clinical and pathological risk factors for recurrence in order to identify the high-risk group for such recurrence and to identify the target group that would benefit from adjuvant chemotherapy. So far, the risk factors universally accepted are emergency surgery under the conditions of cancer perforation or obstruction, low histological grade of tumor and the invasion to other organs, and perivascular, perilymphatic and perineural invasions [19-22]. In addition, patients with conditions that cannot be considered as stage II cancer, i.e., with the number of retrieved or investigated lymph nodes less than 10 to 12, are also included [23]. In this study, on the univariate analysis, patients with T4 tumors (stage IIB/C) showed significantly poor prognosis compared to patients with T3 tumors (stage IIA); the multivariate analysis also showed a poor prognosis even though this result was not statistically significant. However, although it was not described earlier, we could not find any tendency of T4b tumors showing a poorer prognosis than T4a tumors in our population, which was shown in the Surveillance, Epidemiology and End Results (SEER) Report [17]. Additionally, as in existing studies, vascular and neural invasions or bowel obstruction were significant risk factors in this study, and also tumors with lymphatic invasion, the number of obtained lymph nodes less than 12 and poor differentiation showed a tendency to have a poor prognosis, but this result was statistically insignificant.
Determination of risk factors based on such clinical, pathological factors could be quite subjective and could be produce diversified results depending on the reporters, so a need to develop more objective and reproducible risk factors has emerged, and various molecular biomarkers have been studied accordingly. Among them, the most representative one is the presence of a microsatellite instability (MSI), and in this case, adjuvant chemotherapy is not recommended because the MSI-high (H) group presents a good prognosis in the absence of any other risk factors [23]. To this end, some researchers classify cases of any MSI status with the aforementioned clinical and pathological risk factors as the high-risk group, while classifying cases of MS stable (S) with no risk factors as the average risk group and cases of MSI-H with no risk factors as low risk group [24]. Along with them, 18q loss of heterozygosity (LOH) [25] and mutation of KRAS, BRAF genes [26] are being studied as risk factors, but in this study, analyses of such molecular biomarkers were not performed due to the limits of a retrospective study, so such analyses will be performed in future.
This study has limits as a retrospective study in that the factors likely to affect survival, such as the age and the T4b, which showed poor survival results in the SEER Report [17], were not evenly distributed between the treatment and control group. Also, there are limits in accepting the results of this study as the number of patients in the non-treatment group was much lower than the number in the treatment group as most patients with stage II colon cancer received adjuvant chemotherapy according to our hospital policy. Nonetheless, the results of this study seem to have value as domestic data because there have been few reports on the effectiveness of adjuvant chemotherapy only in patients with stage II colon cancer in Korea so far [27, 28].
In this study, the 5-year disease-free survival rate and the 5-year overall survival rate of patients with stage II colon cancer after adjuvant chemotherapy were 87.9% and 92.8%, respectively. This result was found to be statistically significant for both the high- and the low-risk group in comparison to the surgery-only group. In the multivariate analysis, adjuvant chemotherapy was found to be independent prognostic factor that affected overall survival. In the future, prospective multicenter studies addressing the cost benefit of, the risk benefit of, and the choice of an appropriate adjuvant chemotherapy regimen in the treatment of stage II colon cancer patients will be required.
Notes
No potential conflict of interest relevant to this article was reported.