Clinical Significance of Serial Serum Carcinoembryonic Antigen Values for Treating Rectal Cancer with Preoperative Chemoradiotherapy
Article information
Abstract
Purpose
Preoperative chemoradiotherapy is now widely accepted to treat rectal cancer; however, the prognosis for rectal cancer patients during and after chemoradiotherapy must be determined. The aim of this study was to evaluate the serial serum carcinoembryonic antigen (s-CEA) samples in patients with rectal cancer who underwent radical surgery after concurrent chemoradiotherapy (CRT).
Methods
This study evaluated 236 patients with rectal cancer who received preoperative CRT followed by curative surgery between June 2005 and June 2010. We measured the patient's s-CEA levels pre-CRT, post-CRT and post-surgery. Patients were classified into four groups according to their s-CEA concentrations (group 1, high, high, high; group 2, high, high, normal; group 3, high, normal, normal; group 4, normal, normal, normal). We analyzed the clinicopathologic factors and the outcomes among these groups.
Results
Of the 236 patients, 12 were in group 1, 31 were in group 2, 67 were in group 3, and 126 were in group 4. The 3-year disease-free survival rate in group 1 was poorer than those in group 3 (P = 0.007) and group 4 (P < 0.001). In a univariate analysis, type of surgery, clinical N stage, pathologic T or N stage, lymphovascular invasion, perineural invasion, and CEA group were prognostic factors. A multivariate analysis revealed that type of surgery, pathologic T stage, and lymphovascular invasion were independent prognostic factors; however, no statistical significance was associated with the CEA group.
Conclusion
High pre-CRT, post-CRT, and post-surgery s-CEA levels in patients with rectal cancer were associated with high rates of systemic recurrence and poor survival. Therefore, patients with sustained high s-CEA levels during CRT require careful monitoring after surgery.
INTRODUCTION
Preoperative concurrent chemoradiotherapy (CRT) has become widely accepted for the management of rectal cancer. Preoperative CRT for rectal cancer reduces the local recurrence, reduces toxic effect such as diarrhea and bowel stricture, reduces the tumor size, stabilizes the tumor margin to increase resectability, increases the rate of anal sphincter preservation, and reduces tumor metastasis by eliminating local lymph nodes and blocking metastasis routes [1-3].
Several tumor markers are available to predict the response of patients to preoperative CRT and radical surgery. Among these, serum carcinoembryonic antigen (s-CEA) is currently the most widely used tumor marker for managing patients with colorectal cancer [4]. Several studies have shown that measuring s-CEA by tracking the concentration in patients who underwent radical surgery after CRT is helpful in making a diagnosis and in confirming disease recurrence [5-7]. Pre-CRT s-CEA levels may be a useful tool for predicting the response to CRT, and changes in s-CEA before and after CRT might function as an independent prognostic factor in patients with locally advanced rectal cancer [8]. We evaluated the s-CEA levels pre-CRT, post-CRT, and post-surgery in rectal cancer patients and analyzed the disease-free survival (DFS) rates and the prognostic factors in six s-CEA groups.
METHODS
Medical data on 309 patients with rectal cancer who received preoperative CRT followed by curative surgery between June 2005 and June 2010 at Chonnam National University Hwasun Hospital were analyzed retrospectively. The exclusion criteria were patients who did not finish CRT, failed to undergo radical excision, had a metastasis at diagnosis, had another malignancy during the follow-up period, or underwent transanal local excision; 73 patients were excluded based on these criteria. The medical data on the remaining 236 patients were analyzed. A normal s-CEA level is <5 ng/mL. The s-CEA levels, which were measured 1 week before CRT (pre-CRT), 4 weeks after CRT (post-CRT), and 1 week after surgery (post-surgery), were used to assign the patients to one of six groups: group 1, high, high, high; group 2, high, high, normal; group 3, high, normal, normal; group 4, normal, normal, normal; group 5, normal, high, high; group 6, normal, normal, high.
All eligible patients with middle or lower rectal cancer, more clinical T3 stage, or positive clinical N stage underwent preoperative CRT. Although having less than clinical T3 stage or negative clinical N stage, young patients underwent preoperative CRT to preserve the anal sphincter patients. Radiation therapy was executed either as 2- or 3-dimensional conformal therapy using high-energy X-rays (6 or 10 MV). The daily radiation dose was 180 cGy, five times per week for 5 weeks for a total of 4,500 cGy, with conventional fractionation encompassing the rectum and intrapelvic lymph node. A dose of 540 cGy was added to the primary and the high-risk areas after reducing the boost field, projecting a total of 5,040 cGy. Chemotherapy was executed by continuously injecting 5-fluorouracil (500 mg/m2/day) and leucovorin (20 mg/m2) during the first and final weeks of radiotherapy. One round of chemotherapy was conducted in 5 days; two rounds were conducted in total [9].
A digital rectal examination, blood chemistry, s-CEA assay, simple chest X-ray, abdominopelvic computed tomography (CT), pelvic magnetic resonance imaging (MRI), positron emission tomography (PET), colonoscopy, and biopsy were executed as baseline tests. Surgery was conducted 6 to 8 weeks after CRT. High ligation and total mesorectal excision were set as the bases in all patients. Histology was categorized based on the results of a colonoscopic biopsy and was recorded as well differentiated, moderately differentiated, poorly differentiated, and mucinous differentiated. Additionally, perineural and lymphovascular invasions were assessed based on the postoperative pathologic findings. Follow-up examinations after surgery were done every three months during the first two years and every six months after that. The examinations included a digital rectal exam, laboratory tests, s-CEA assay, chest X-ray and abdominopelvic CT. Chest CT, pelvic MRI, and PET scans were executed based on need. Local recurrence was defined as the presence of a histologically-proven or a radiologically-confirmed tumor in the pelvic cavity or anastomosis within the field of surgery. Systemic recurrence was defined as distant solid organic metastasis, systemic positive lymph nodes, or peritoneal seeding.
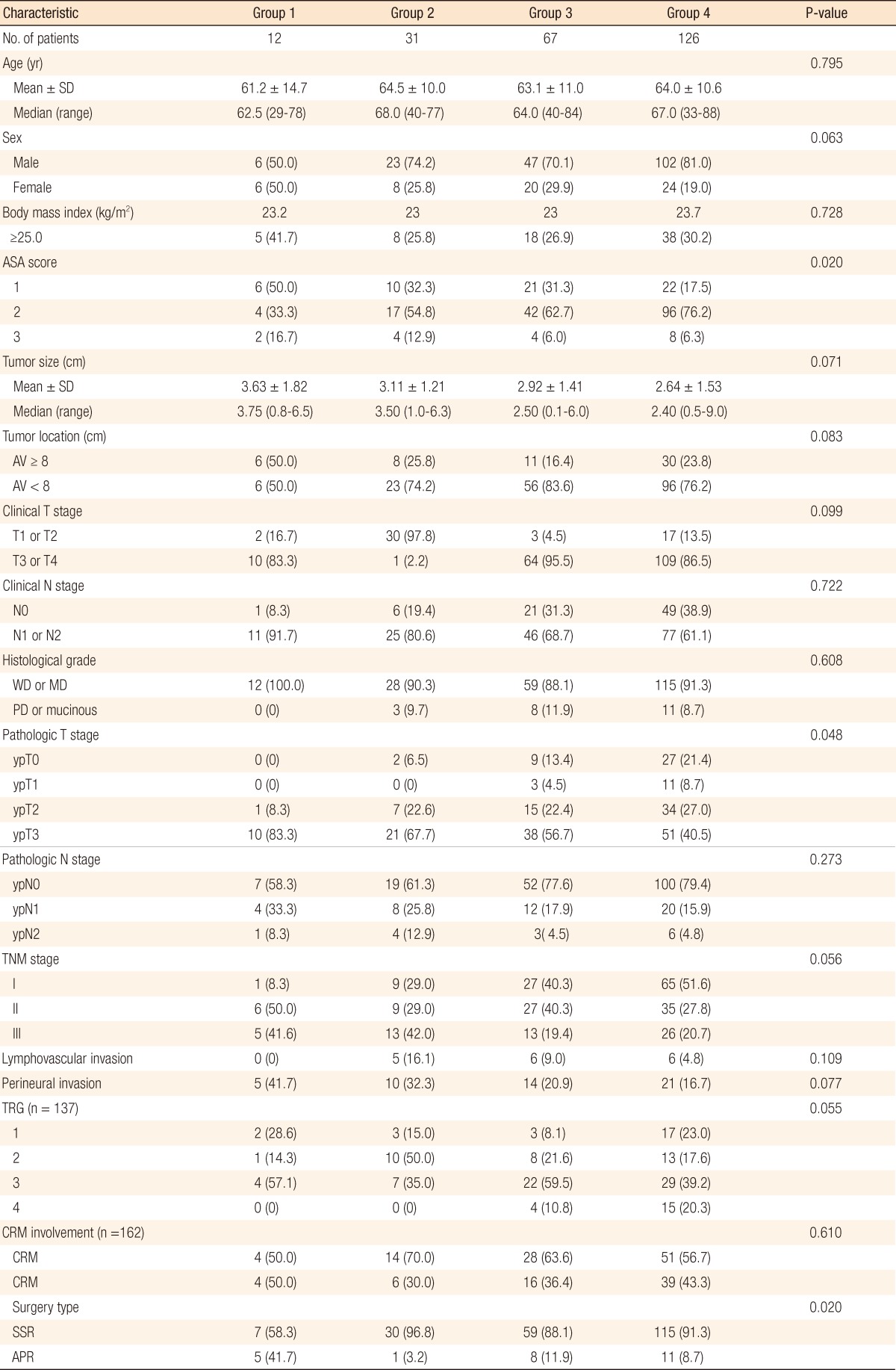
We measured age, sex, body mass index, American Association of Anaesthetists (ASA) score, tumor size, tumor location, clinical stage, pathological stage, histological grade, lymphovascular invasion, perineural invasion, tumor regression grade (TRG), circumferential resection margin (CRM) involvement, and surgery type among the groups. Residual tumor mass after preoperative treatment was semi-quantitatively evaluated according to Dworak et al. [10] 5-point grading system for tumor regression. A CRM-positive tumor was defined as a tumor at the CRM or a tumor with a minimal distance between the tumor and the CRM of ≤1 mm. A CRM-negative tumor was defined as a tumor with a minimum distance between the tumor and the CRM >1 mm [11]. Pathological stage was evaluated based on the categories set forth by 2010 American Joint Committee on tumor-node-metastasis classification, 7th edition.
Of the 236 patients, 214 (90.7%) received adjuvant chemotherapy. FL (5-fluorouracil [5-FU]/leucovorin, six cycles of monthly bolus intravenous 5-FU 400 to 425 mg/m2/day, days 1 to 5 and leucovorin 20 mg/m2/day, days 1 to 5) was administered to 182 (77.1%), FOLFOX (5-FU/leucovorin/oxaliplatin, twelve cycles of monthly bolus intravenous 5-FU 400 to 425 mg/m2/day, days 1 to 2 and leucovorin 20 mg/m2/day, days 1 to 2, and oxaliplatin 85 mg/m2, day 1) to 18 (7.6%), UFT (tegafur/uracil, six cycles of UFT 300 mg/m2/day for 28 days) to 7, and capecitabine (8 cycles of oral 5-FU 2,500 mg/m2 for 2 weeks) to 7 patients. The remaining 22 (9.3%) did not receive chemotherapy because of refusal or poor general condition.
The statistical analysis was done using IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA). Frequency differences in each group were checked using the t-test and the chi-squared test. Survival rates were compared with the Kaplan-Meier method and the logrank test. The Cox proportional hazards model was used for the multivariate analysis. A P-value < 0.05 was considered statistically significant.
RESULTS
Of the 236 patients, 12 were in group 1, 31 were in group 2, 67 were in group 3, and 126 were in group 4. No patients were categorized into group 5 or 6. Male patients dominated (75.4%), with a median age of 64 years. The median follow-up interval was 22 months (range, 1 to 75 months). The ASA score was different between groups. When we compared the clinicopathological characteristics among the four groups, the patients in group 1 were more likely to have an advanced pathologic T stage (P = 0.048) because CRT had little effect on downstaging; they underwent an abdominoperineal resection (APR, P = 0.020). Nevertheless, tumor size, tumor location, clinical T or N stage, histological differentiation, pathologic N stage, lymphovascular invasion, perineural invasion, TRG, and CRM involvement did not differ among the s-CEA groups (Table 1).
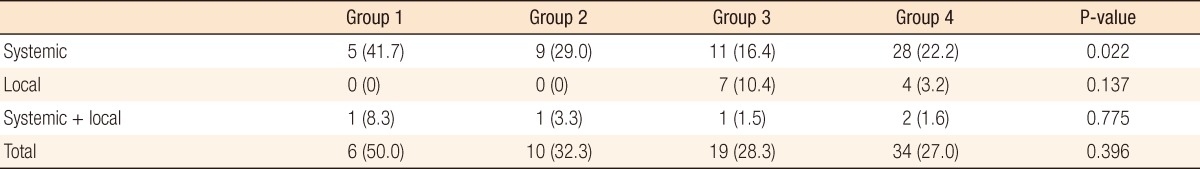
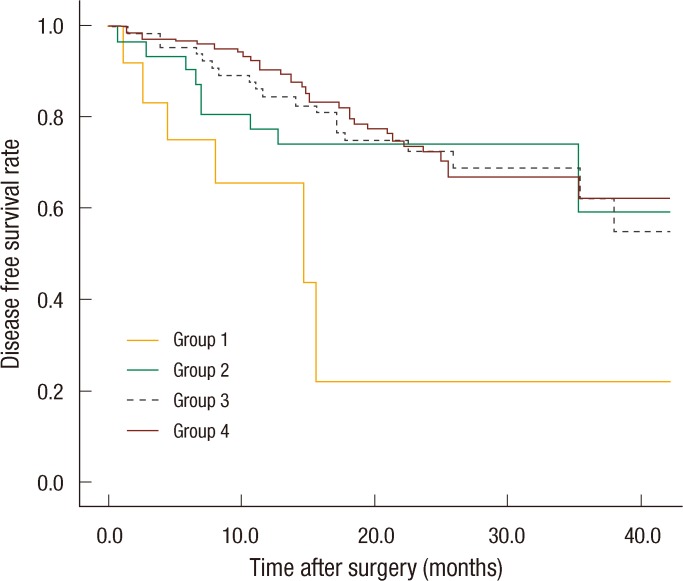
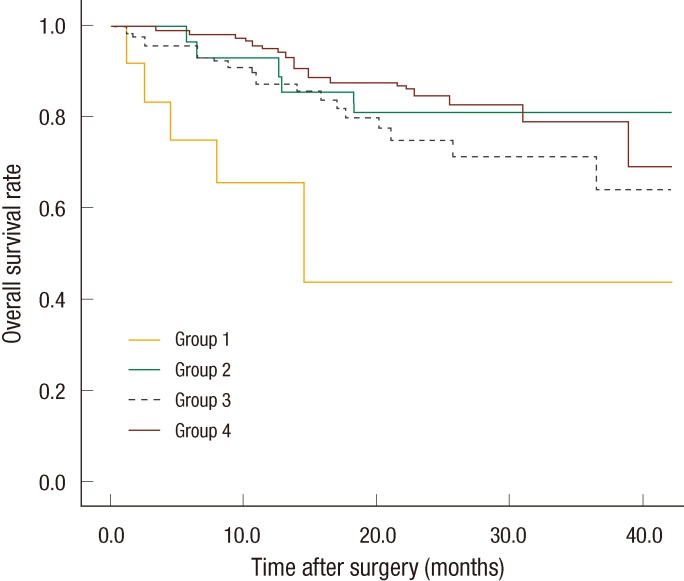
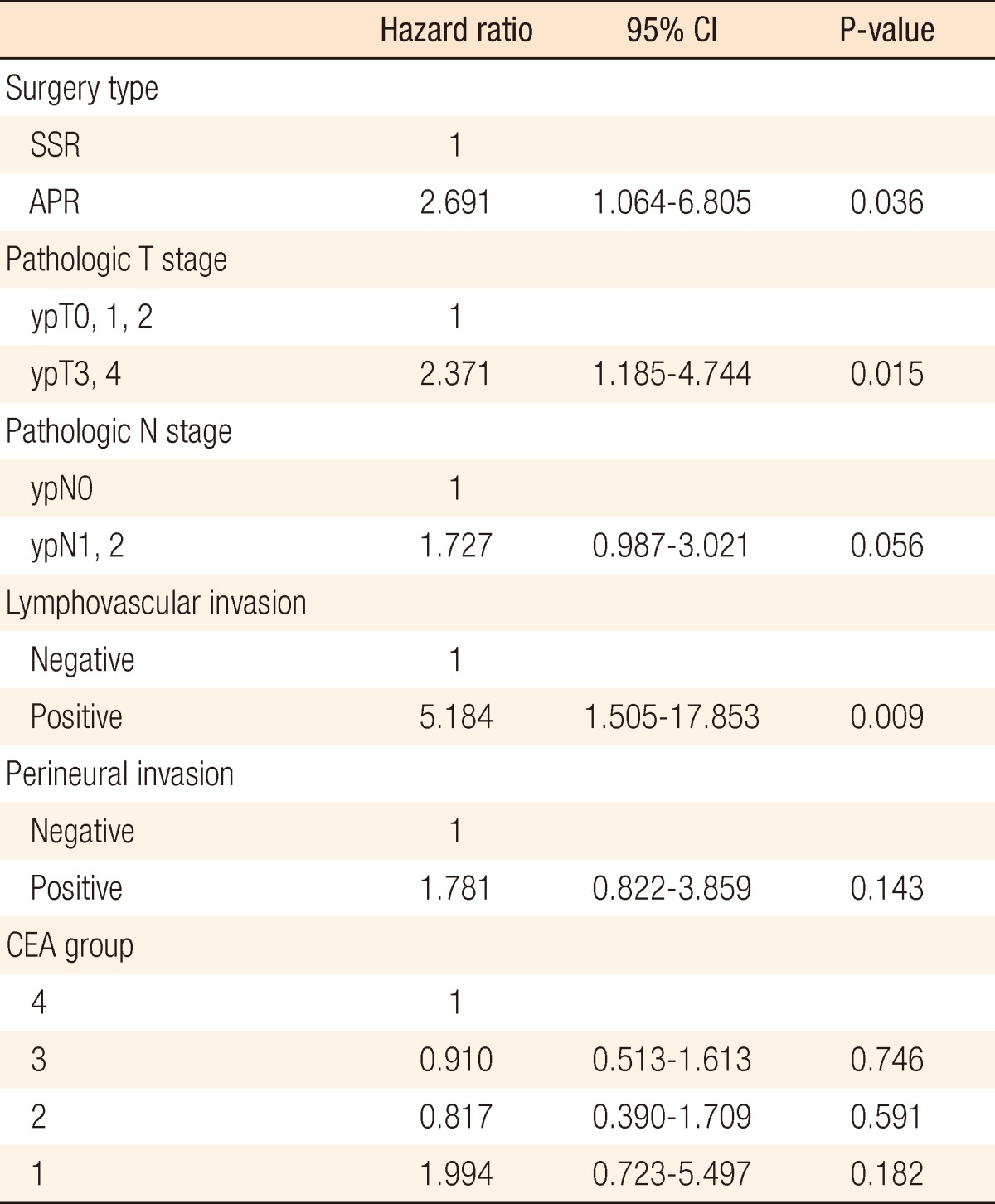
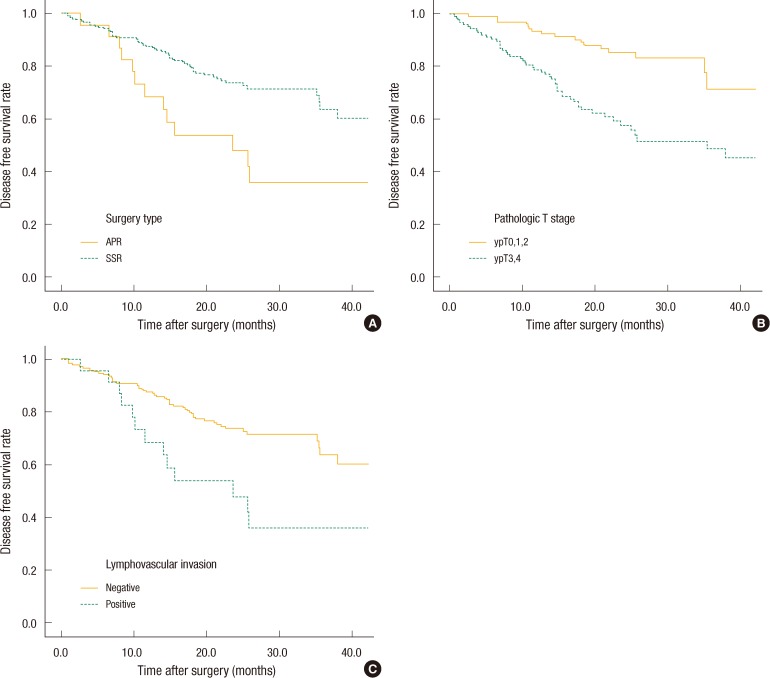
Systemic recurrence occurred frequently in group 1 and was higher than it was in the other groups. The most distant metastatic organ was the lung, followed by the liver. No significant difference in local recurrence was observed among the four groups (Table 2). The 3-year DFS rates of the groups were as follows: group 1, 21.9%; group 2, 59.4%; group 3, 69%; group 4, 62.2%. No significant differences were found between groups 1 and 2 (P = 0.093), but a significant difference was observed between groups 1 and 3 (P = 0.007) and between groups 1 and 4 (P < 0.001). No significant differences were observed between groups 2 and 3 (P = 0.638), between groups 2 and 4 (P = 0.587), or between groups 3 and 4 (P = 0.872) (Fig. 1). The 3-year overall survival (OS) rates of the groups were as follows: group 1, 43.7%; group 2, 81.1%; group 3, 77.6%; group 4, 79.1% (Fig. 2). In the univariate analysis for the prognosis, surgery type (Sphincter-sparing resection vs. APR, P = 0.006), clinical N stage (P < 0.001), ypT-stage (P < 0.001), ypN-stage (P < 0.001), lymphovascular invasion (P < 0.001), perineural invasion (P < 0.001), and CEA group (P = 0.021) were prognostic factors for DFS (Table 3). However, a multivariate analysis revealed that type of surgery (odds ratio [OR], 2.691; P = 0.036), ypT stage (OR, 2.371; P = 0.015), and lymphovascular invasion (OR, 5.184; P = 0.009) were statistically significant prognostic factors (Table 4, Fig. 3).
DISCUSSION
Since the first description of s-CEA, this marker has been the most widely used and readily available tumor marker for managing patients with colorectal cancer. The measurement of s-CEA has been standardized, is easily performed, and is inexpensive compared to other molecular and biochemical tumor marker. Most studies regarding s-CEA in patients with colorectal cancer have focused on the prognostic effect of the preoperative s-CEA concentration, and elevated preoperative s-CEA levels have been related to high recurrence rate [5, 12-15].
Known prognostic factors of rectal cancer include tumor size, histological grade, node metastasis, and lymphatic or venous invasion [6, 16]. A lack of association between pathological stage and tumor recurrence in patients with rectal cancer treated with preoperative radiation or chemoradiation has been reported [17, 18]. The patients in group 1 in the present study had an advanced T stage because CRT had little effect on downstaging; they also had a tendency to undergo an APR. Chen et al. [19] reported a higher local recurrence rate in patients after an APR compared to those who underwent a sphincter-sparing resection, but surgery type was not identified as an independent risk factor for survival [19].
The CRM status is a major factor in assessing the quality of the surgery and is a powerful prognostic factor; however, a high frequency of CRM-positive patients was shown in present study, which is associated with the distribution of lower rectal cancer or high stage. Of the CRM-positive patients, 68 patients had a CRM status of 1 mm on the pathologic results.
Pre- and post-CRT s-CEA levels as predictors of response or as prognostic factors in patients with rectal cancer who receive preoperative CRT, as well as a reduction in preoperative s-CEA level after radical surgery, have been associated with improved disease-free and OS in patients with rectal cancer [20-23]. Wang et al. [24] revealed that preoperative and postoperative s-CEA levels were independent predictors of survival and recurrence. Lin et al. [25] reported that early postoperative s-CEA levels compared to preoperative s-CEA levels were a better prognostic indicator in patients with curable colorectal cancer. Park et al. [6] recognized three s-CEA groups based on their preoperative and early postoperative s-CEA levels. They revealed that patients with rectal cancer who had increased preoperative and early postoperative s-CEA levels showed more frequent systemic failure recurrences and a worse survival rate. In our study, the systemic recurrence rate in group 1 was significantly higher than that in the other groups. Although no correlation with CEA group was identified in the multivariate analysis, the differences in DFS and OS between group 1 and groups 2 to 4 had statistically significance (P = 0.003, P = 0.001).
The limitations of this study include a relatively-short follow-up period and a small number of specific groups. Additional studies are needed to evaluate the possibility of using serial s-CEA levels as a tool for monitoring the response in patients with rectal cancer who underwent preoperative CRT and radical surgery.
In conclusion, the continuous monitoring of s-CEA levels during CRT and surgery reflected the prognosis for and the frequency of systemic recurrence in patients with rectal cancer. Sustained high s-CEA levels, which means group 1 in this study, during CRT and surgery resulted in high systemic recurrence, as well as poor DFS and OS rates. High s-CEA levels during CRT and surgery were prognostic factors poor outcomes; thus, patients with such levels require careful monitoring after surgery.
Notes
No potential conflict of interest relevant to this article was reported.