Prognostic Implication of 15-Hydroxyprostaglandin Dehydrogenase Down-Regulation in Patients with Colorectal Cancer
Article information
Abstract
Purpose
Prostaglandin (PG) E2 is known to be closely related to cancer progression and is inactivated by 15-hydroxyprostaglandin dehydrogenase (PGDH). 15-PGDH is shown to have tumor suppressor activity and to be down-regulated in various cancers, including colorectal cancer (CRC). Therefore, we evaluated the expression of 15-PGDH and its prognostic effect in patients with CRC.
Methods
15-PGDH expression was examined by using immunohistochemistry in 77 patients with CRC. Its prognostic significance was statistically evaluated.
Results
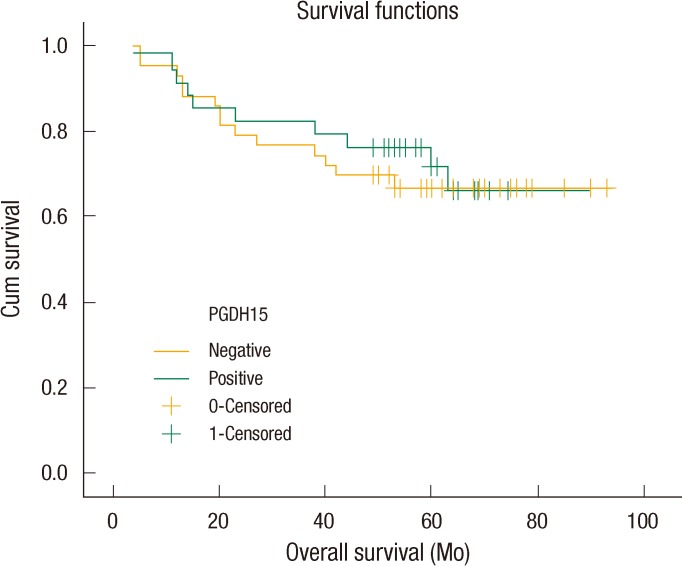
Negative 15-PGDH expression was noted in 55.8% of the 77 cases of CRC. 15-PGDH expression showed no correlation with any of the various clinicopathologic parameters. The status of lymph node metastasis, tumor-node-metastasis stages, and pre-operative carcinoembryonic antigen levels showed significant prognostic effect. However, univariate analysis revealed down-regulation of 15-PGDH not to be a predictor of poor survival. The 5-year overall survival rate was 71.7% in the group with positive expression of 15-PGDH and 67.1% in the group with negative expression of 15-PGDH, but this difference was not statistically significant (P = 0.751).
Conclusion
15-PGDH was down-regulated in 55.8% of the colorectal cancer patients. However, down-regulation of 15-PGDH showed no prognostic value in patients with CRC. Further larger scale or prospective studies are needed to clarify the prognostic effect of 15-PGDH down-regulation in patients with colorectal cancer.
INTRODUCTION
The adenoma-carcinoma sequence and its related genes was reported in the carcinogenesis of colorectal cancer in the 1990s [1]. However, more molecular biological mechanisms have been clarified in recent years. Several pathways are known to be involved in the process of colorectal carcinogenesis, including the chromosomal instability pathway, the CpG island methylator phenotype pathway, the microsatellite instability pathway, and the serrated pathway [2]. In addition, chronic inflammations may play a crucial role in cancer initiation. As proof, the incidence of colorectal cancer has been shown to be greater in patients with inflammatory bowel disease [3]. Prostaglandin (PG) is identified as a substance that is profoundly related to inflammatory response. PGs are bioactive lipids produced from arachidonic acid converted from the metabolism of phospholipids in membranes, including PGE2, PGD2, PGF2α, PGI2, and thromboxane A2. They play crucial roles in physiological aspects such as renal function maintenance, gastrointestinal function maintenance, and the regulation of vascular homeostasis, as well as, during the inflammatory response [4].
Among the different PGs, PGE2 is especially known to promote carcinogenesis and cancer progression by activating cancer cells, promoting angiogenesis, and inhibiting apoptosis [5]. Cyclooxygenase (COX) plays the most significant role in PGE2 production. There are mainly two types of COX. COX-1 is involved in normal physiological function by maintaining a constant amount while COX-2 is expressed in an increased amount induced by certain stimulation. Inflammatory response is known to be involved in the formation of PGE2 [6]. Moreover, COX-2 is known to play a role as an oncogene, and COX-2 is over-expressed in various solid tumors, including colorectal cancer. Pharmacological inhibitors of COX, such as aspirin, sulindac and celecoxib, have been demonstrated to lower the incidence of colon cancer, gastric cancer, and esophageal cancer, and the use of these inhibitors has been shown through epidermiologic studies to lower the mortality rates of these cancers [7]. However, the long-term use of COX-2 suppressors is known to have serious adverse effects on the gastrointestinal and cardiovascular systems. The causes are thought to be that COX-2 suppressors not only inhibit PGE2 production but also inhibit the formation of other various PGs at the same time [8]. Therefore, studies on substances that can selectively reduce PGE2 only are essential. Microsomal prostaglandin E synthase is currently known to overcome the limitations of COX-2 and to involve the synthesis of PGE2 selectively. Furthermore, 15-hydroxyprostaglandin dehydrogenase (15-PGDH) is known as enzymedegrading 15-PGDH [9]. Because these enzymes are involved in the metabolism of PGE2 by working at the lower phase of COX-2, they are anticipated to regulate PGE2 selectively and avoiding the adverse effects of COX-2.
15-PGDH is an enzyme that converts PGE2 into 15-keto-prostaglandin by working on 15(S)-hydroxyl groups, eventually leading to inactivation of PGE2. Recent studies have demonstrated that decreased 15-PGDH has a profound relationship with carcinogenesis and cancer progression. Decreased 15-PGDH expression was observed in patients with colorectal cancer, breast cancer, prostate cancer, lung cancer, thyroid cancer, gastric cancer and other cancers [10, 11]. Moreover, Myung et al. [12] reported an increase in colon tumorigenesis in 15-PGDH knock-out mice and proposed that 15-PGDH played the role of a tumor suppressor. The authors of this study reported that the expression of 15-PGDH was lower in colorectal cancer and that it was inversely correlated with the expression of vascular endothelial growth factor (VEGF), which is closely correlated with angiogenesis [13]. Such a result suggested a significant relationship between down-regulation of 15-PGDH and cancer progression. As the role of 15-PGDH as a tumor suppressor has been proven, a hypothesis that down-regulation of 15-PGDH will work as a poor prognostic parameter can be formulated. However, few studies have been carried out on the role of 15-PGDH as a prognostic predictor for colorectal cancer. Therefore, this study aims to investigate the role of 15-PGDH expression in colorectal cancer patients as a prognostic parameter.
METHODS
Patients and tissues
Seventy-seven patients who had undergone surgical resection for a primary sporadic colorectal carcinoma by a single colorectal surgeon at the Department of Surgery, Chosun University Hospital, between March 2002 and December 2005 were included in this study. The study excluded patients who regularly took aspirin or other anti-inflammatory drugs, those who underwent preoperative chemotherapy or radiotherapy, and those who had evidence of hereditary or familial CRC syndrome. Enrolled patients were followed up until death or December 2008. The mean follow-up period was 52.5 months (4 to 93 months), and the mean age of the patients was 63.9 (±11.4) years old at the time of surgery. Surgically-resected tissues were classified by pathologists according to their characteristics, including histological differentiation, the depth of tumor invasion, lymph node invasion, the presence of distant metastasis, etc. In all cases, archived hematoxylin and eosin-stained tissue slides were obtained for confirmation of pathological features and for the selection of suitable tissue blocks for immunohistochemical analysis.
Immunohistochemical staining
The expression of 15-PGDH was observed in resected colorectal cancer tissue by immunohistochemical staining. Paraffin blocks were cut into 4-micrometer-thick pieces and washed after the paraffin had been removed with xylene and alcohol. Subsequently, sections were immersed in sodium citrate buffer (pH 6.0) and autoclaved at 121℃ for 15 minutes for antigen retrieval. In addition, they were treated in methanol with 3% hydrogen peroxide for 20 minutes in order to block the activation of endogenous peroxidase. Slides were treated with Ultra V Block (UltraVision Plus Detection System, Thermo Fisher Scientific Inc., Fremont, CA, USA) solution at room temperature for five minutes to inhibit non-specific binding. Rabbit polyclonal antibody (1:2,000, Novus Biologicals, Littleton, CO, USA) was used as the 15-PGDH primary antibody and was incubated at 4℃ overnight. Each slide was washed with phosphate buffered saline (PBS) four times and incubated with biotinylated goat anti-rabbit antibody (UltraVision Plus Detection System) at room temperature for five minutes. Afterward, each slide was washed again with PBS four times and incubated with streptavidin-alkaline phosphate conjugate (UltraVision Plus Detection System). Moreover, each slide was washed four times repeatedly with PBS and developed with Fast Red tablets on a naphthol phosphate substrate. Slides were counterstained with Mayer's hematoxylin solution for 20 seconds and air-dried and enclosed with a cover slip. Normal rabbit serum immunoglobulin G (Vector Laboratories Inc., Burlingame, CA, USA) was taken as a negative control, replacing the primary antibody. The mucosa of a normal colon was used as an internal control. All our experiments were performed in duplicate.
Determination of immunohistochemical staining
Each slide was assessed by pathologists who were unaware of the patients' clinical information. A patient was defined as being positive when 15-PGDH was expressed in more than 30% of the cancer cells of each tissue sample. In contrast, a patient was defined as being negative or down-regulated when 15-PGDH was expressed in less than 30% of those cells [14].
Statistical analysis
The relationship between 15-PGDH expression and clinical manifestation was analyzed using a chi-square test and Fisher's exact test. Patients' survival rates were analyzed using the Kaplan-Meier method. The differences in survival rates were examined using a log-rank test. P-values of less than 0.05 were defined as statistically significant. SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA) was used for data analysis.
RESULTS
The expression of 15-PGDH was observed in normal mucosa of all colorectal cancer patients. Expression of 15-PGDH was down-regulated in 34 (55.8%) out of 77 patients (Fig. 1). The expression rates of 15-PGDH were 50% and 40% in colon and rectal cancers, respectively. However, no statistical difference was found between the two groups. Down-regulation of 15-PGDH was revealed in the patient group with increased preoperative carcinoembryonic antigen (CEA) levels, indicating a statistically marginal significance (P = 0.056). No differences were detected in 15-PGDH expression depending on the depth of tumor invasion (T-stage), lymph node invasion (N-stage), the presence of distant metastasis (M-stage), etc (Table 1).
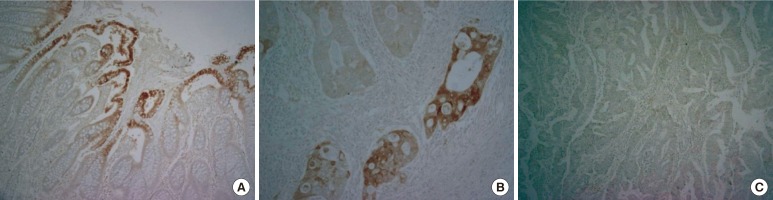
Immunohistochemistry for 15-prostaglandin dehydrogenase (15-PGDH) in normal colon tissues and in colorectal cancer tissues: (A) normal 15-PGDH expression in colon mucosa (×100), (B) positive 15-PGDH expression in cancer tissue (×200), and (C) negative 15-PGDH expression in cancer tissue (×100).
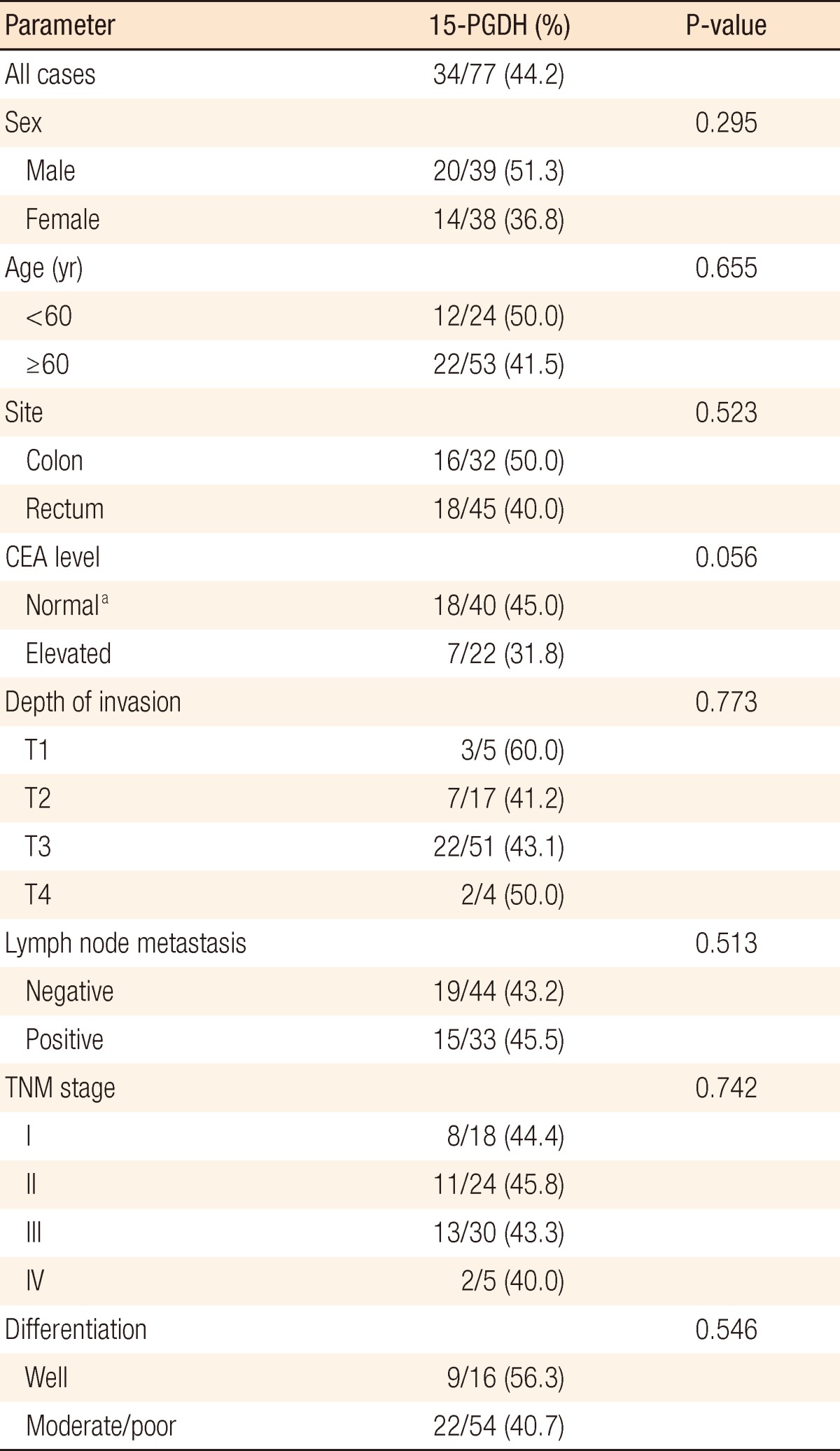
Correlation of 15-prostaglandin dehydrogenase (15-PGDH) expression with clinicopathologic parameters
The study analyzed various clinicopathologic factors affecting the survival rate of patients with colorectal cancer. Long-term survival was anticipated in the groups with no lymph node metastasis, low tumor-node-metastasis (TNM) stages, and normal preoperative CEA levels (Table 2). When the survival analysis was performed using a Kaplan-Meier analysis, the 5-year survival rates were 88.9% at stage I, 79.2% at stage II, 57.9% at stage III and 20% at stage IV, showing statistical significance (P < 0.001). Moreover, significant differences were observed in the disease-free survival rate depending on TNM stage (P = 0.033, data not shown).
The five-year survival rates were 71.7% in patients with 15-PGDH expression and 67.1% in patients with down-regulation of 15-PGDH, but this difference was not statistically significant (Fig. 2). The difference in survival rates of COX-2-positive patients were analyzed depending on the status of 15-PGDH expression because 15-PGDH, as well as COX-2, plays a significant role in relation to PGE2 metabolism. The five-year survival rates of COX-2 positive patients were 73.3% in 15-PGDH-positve patients and 68.9% in 15-PGDH-negative patients, but this difference was not statistically significant (data not shown).
DISCUSSION
The authors of the study intended to investigate the prognostic implication of 15-PGDH, which is known for its role as a tumor suppressor gene, for patients with colorectal cancer. However, statistical significance was not detected. NAD(+)-dependent 15-PGDH with a molecular weight of 29 kDa is involved in PGE2 metabolism, and it is known to be activated as a homodimer [15].
Yan et al. [16] verified that 15-PGDH mRNA and protein were both highly expressed at the normal colon mucosa while almost no expression was assessed in colon cancer tissues and that xenograft tumor formation of colon cancer cells was lower in nude mice when 15-PGDH was transfected. Thus, they suggested the effects of 15-PGDH expression as a tumor suppressor in colon cancer. During a similar period, Backlund et al. [17] reported that the activity and the expression of 15-PGDH was lower in colon cancer cells, breast cancer cells, lung cancer cells and the adenomas in Apc Min mouse and that they were higher in normal colon tissues of humans or mice. Myung et al. [12] examined the role of 15-PGDH as a tumor suppressor gene in vivo. Moreover, 15-PGDH gene knockout in an Apc Min mouse model increased the incidence of tumors in the mouse colon by a factor of 7.6. They suggested that 15-PGDH played the role of a tumor suppressor by using an in vivo experiment in which C57BL/6J rats were administered the carcinogen azoxymethane to demonstrate an increased incidence of adenomas or intraepithelial carcinomas in 15-PGDH gene knock-out mice. In previous studies, we also found that 15-PGDH was down-regulated in the tissues of colorectal cancer patients and that the expressions of 15-PGDH and VEGF were inversely correlated, suggesting a tumor progression effect of 15-PGDH down-regulation in colorectal cancer [13]. Besides colorectal cancer, 15-PGDH is known for its role as a tumor suppressor gene in various other cancers. The expression of 15-PGDH is markedly lower in lung cancer tissues compared to adjacent normal pulmonary tissues. In conjunction with up-regulation of COX-2 and PGE synthase, down-regulation of 15-PGDH increased PGE2 levels, consequently causing proliferation of a tumor [18]. Down-regulation of 15-PGDH was detected in around 40% of breast cancer tissues and is reported to be correlated with the expression of the estrogen receptor. When 15-PGDH was transfected in breast cancer cells, the tumor formation rate was lower in rats [19]. According to the expression of 15-PGDH in gastric cancer, 15-PGDH mRNA was five times lower in cancer tissues compared to adjacent normal tissues. 15-PGDH protein was down-regulated in 65% of the patients. Wolf et al. [19] also reported that the mechanism of 15-PGDH down-regulation was methylation in the promoter region of 15-PGDH [20]. Kaliberova et al. [21] attempted gene therapy on nude mice by transfecting 15-PGDH to breast cancer cells (2LMP) and colon cancer cells (LS174T). The gene therapy of 15-PGDH delayed the tumor growth in both breast cancer cells and colon cancer cells. When combined 15-PGDH gene therapy and bevacizumab were concurrently applied to LS174T cells, tumor growth declined significantly compared to the sole use of bevacizumab.
Although the tumor suppressor activity of 15-PGDH has already been verified by many studies, only a few studies have investigated the role of 15-PGDH for its prognostic implications [21-23]. Tatsuwaki et al. [22] reported that decreased expression of 15-PGDH was observed in 35 out of 71 gastric cancer patients and that it was an independent adverse prognostic parameter. On the other hand, Thiel et al. [20] reported that 15-PGDH expression status in gastric cancer tissues had no significant relationship with prognostic implication. Lehtinen et al. [23] paradoxically reported that high expression of 15-PGDH mRNA was correlated with poor prognosis in 295 patients with breast cancer. Although the present study identified that the five-year survival rate was slightly higher when 15-PGDH was expressed, no statistical difference was detected when 15-PGDH expression was used as a prognostic parameter.
The authors think this study is worthy of note as the first research to investigate the role of 15-PGDH as a prognostic parameter in colorectal cancer. Prospective studies with a larger number of study subjects are thought to be essential because the results on the role of 15-PGDH as a prognostic parameter differ extensively from study to study and are often contradictory.
In conclusion, in this study, 15-PGDH was down-regulated in 55.8% of the patients with colorectal cancer. No significant correlation was detected between the expression of 15-PGDH and the prognostic implications for patients with colorectal cancer. However, further studies with larger study populations are thought to be crucial because this study was limited to a small number of subjects.
ACKNOWLEDGMENTS
This research was supported by a grant from Chosun University in 2008.
Notes
The main idea of this research was presented orally at the Korean Society of Coloproctology in 2011.
No potential conflict of interest relevant to this article was reported.