Lateral Lymph Node Dissection With the Focus on Indications, Functional Outcomes, and Minimally Invasive Surgery
Article information
Abstract
The lateral lymph node dissection (LLND) is still a subject of great debate as to the appropriate treatment for patients with mid to low advanced rectal cancer. The guidelines of the Japanese Society for Cancer of the Colon and Rectum recommend a LLND for patients with T3/4 rectal cancer below the peritoneal reflection. However, in most Western countries, a routine LLND is not recommended unless a node or nodes are clinically suspicious for metastasis. Even after preoperative chemoradiotherapy (CRT), an 8% to 12% lateral pelvic recurrence was noted. The size of the lateral lymph node and responsiveness to preoperative CRT should be the main factors for selecting appropriate patients to undergo a LLND. In addition, from the recent literature, a laparoscopic LLND is safe and oncologically feasible and might have some advantages in short-term outcomes.
INTRODUCTION
The incidence of lateral pelvic lymph node metastasis has been estimated to range from 11% to 22% in patients with T3/4 rectal cancer below the peritoneal reflection [1-5]. Although the therapeutic benefit of lateral lymph node dissection (LLND) is reported to be higher than that of lymph node dissection in the area of either the superior rectal or the inferior mesenteric artery [6], the strategy for the use of a LLND has varied among nations and surgeons because of different views concerning lateral pelvic lymph node metastasis. In the view of Western countries, preoperative chemoradiotherapy (CRT) followed by a total mesorectal excision (TME) has been the standard treatment for patients with locally advanced rectal cancers, and metastasis to extramesorectal lymph nodes, except the lymph nodes in the area of the internal iliac artery, has been regarded as a systematic disease that requires systemic chemotherapy [7, 8]. However, in recent reports, preoperative CRT with a TME could not completely render the lateral lymph node benign, so the remnant metastatic lymph node could still cause local pelvic recurrence [9, 10]. In Japan, surgeons have been using the LLND since the 1970s to decrease local recurrence and improve prognosis [11], and the procedure has progressed from a routine en bloc extended pelvic lymphadenectomy to autonomic nerve-preserving surgery; furthermore, selective application of the procedure has reduced functional compromise and increased its oncologic efficacy [12-15].
In this paper, we will review the indications for selective LLND in the era of preoperative CRT because preoperative evaluation has progressed due to the adoption of high-resolution rectal magnetic resonance imaging (MRI) and to the fact that more reliable imaging of the lateral pelvic lymph node has become possible. We will also review the recent literature on the LLND from the perspective of functional outcomes and the setting for minimally invasive surgery.
INDICATIONS FOR A LATERAL PELVIC LYMPH NODE DISSECTION
Recently, interest in LLND for treating patients with locally advanced rectal cancer has been growing, but the optimal indications for LLND have not yet been established. In Japan, the guidelines of the Japanese Society for Cancer of the Colon and Rectum (JSCCR) recommend that a bilateral LLND be performed in patients whose lower tumor border is located distal to the peritoneal reflection and whose cancer has invaded beyond the muscularis propria. The LLND lowered the risk of intrapelvic recurrence by 50%, and the 5-year survival rate improved by 8% to 9% without preoperative CRT [16].
On the other hand, the National Comprehensive Cancer Network does not recommend an extended lymphadenectomy unless lateral lymph nodes are clinically suspicious, and the detailed indications for LLND have not been clearly defined. However, researchers have recognized that preoperative CRT with TME might not be sufficient to prevent lateral local recurrences [9, 10]. Kim et al. [10] noted that 83% of patients with locoregional recurrence had lateral pelvic recurrence even after preoperative CRT and a curative proctectomy. Kusters et al. [9] reported a 5-year lateral local recurrence rate of 11.8% in the Western population, and patients with lateral nodes with malignant features had a lateral local recurrence rate (20.9%) twice as high as those without malignant-looking nodes (10.3%).
The size of the lateral LN before treatment has been reported to be the main factor for predicting lateral pelvic recurrences and metastasis to lymph nodes. The cutoff size of lateral nodes has been reported to vary from 5 to 8 mm [17-20], but a recent JSCCR report has identified a 5-mm cutoff on the short axis as being optimal for detecting metastatic nodes. The rate of lateral pelvic recurrence increases as the size of the lateral LN increases [10], and when the diameter of the largest lateral LN is in the range from 5 to 10 mm or more than 10 mm, the incidence of lateral spread is 20% or 36.7%, respectively [6]. Based on MRI imaging, JSCCR studied the optimum cutoff for lateral lymph node size to identify metastatic nodes; it found that a 5-mm cutoff on the short-axis was superior to a 10-mm cutoff [18]. It also reported that compared with other factors, including histopathological grade, perirectal nodes, and distant metastasis, an enlarged pelvic node status with a short axis ≥5 mm on MRI was the most important risk factor for metastasis [21].
After administration of preoperative CRT, the responsiveness, that is, the change in LN size, may be considered as another factor indicating the need for LLND. Oh et al. [22] reported that unlike responsive lymph nodes, persistent lateral lymph nodes greater than 5 mm in size after CRT were significantly associated with residual tumor metastasis. Kim et al. [23] divided patients into three groups: group I (no suspicious lateral LNs), i.e., lateral LN < 5 mm pre- and post-CRT; group II (responsive lateral LN), lateral pelvic node ≥5 mm pre-CRT, but <5 mm post-CRT; and group III (persistent lateral pelvic node), lateral pelvic node ≥5 mm pre- and post-CRT. Group III had significantly poorer lateral pelvic node recurrence-free survival than groups I and II. In summary, the size of the lateral LN and the responsiveness to preoperative CRT might be the main factors for considering LLND. Further research is needed to define the optimal indications for LLND.
FUNCTIONAL OUTCOMES AFTER A LATERAL LYMPH NODE DISSECTION
The functional compromise after LLND for patients with rectal cancer has been a major concern (Table 1). The operative extent of LLND involves inevitably some injury to the vessels and nerves in the pelvic sidewall, and the sexual and voiding functions are usually decreased after LLND. In early reports from Japan, LLND included an extended systematic lymphadenectomy, which means para-aortic and paracaval lymphatic dissection from the left renal vein to the aortic bifurcation along the adventitial layers of the inferior vena cave and abdominal aorta [24]. This procedure accompanied inevitable functional deteriorations [25, 26] and increased incidences of urine-voiding failure (39.4% after the extended lymphadenectomy vs. 8.8% after the conventional lymphadenectomy) and sexual impotency (76% vs. 37.5%). In the meta-analysis, the extended lymphadenectomy showed a 3.7 times higher risk of urinary dysfunction and a 2.08 times higher risk of urinary retention [8].
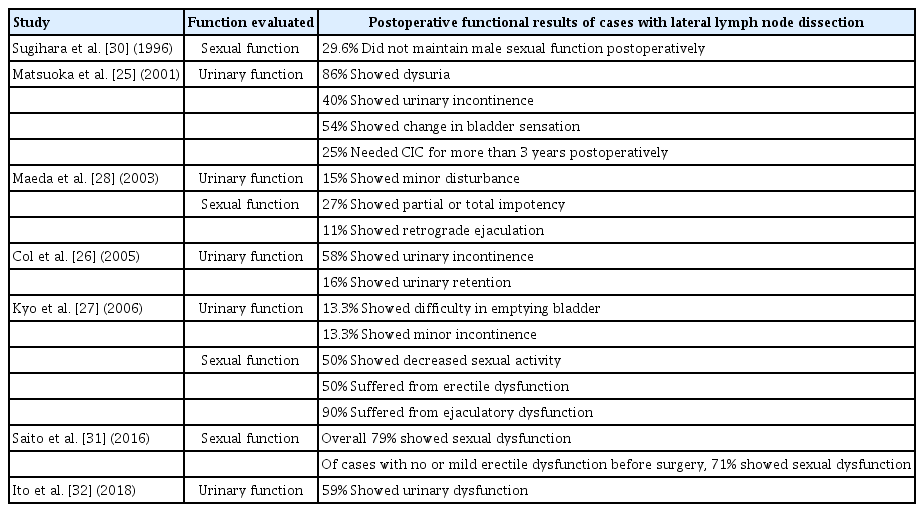
Summary table of literature review regarding postoperative functional outcomes of cases with lateral lymph node dissection
However, the literature on a nerve-preserving lymphadenectomy demonstrates that autonomic nerve preservation offers advantages in maintaining urinary and sexual functions [13, 27-30]. In the recent Japan Clinical Oncology Group 0212 trial, postoperative urinary dysfunction developed in 59% of patients who underwent LLLD, and sexual dysfunction occurred in 79% of such patients; these results were not significantly different from those for the TME-alone group [31, 32]. The degree of dysfunction has been reported to be related to the extent of the lymphadenectomy and autonomic nerve preservation [29], so the oncological benefits and functional deterioration should be balanced.
LAPAROSCOPIC LATERAL LYMPH NODE DISSECTION
Laparoscopic LLND with its enhanced visualization may be the next promising approach and may not only provide a survival benefit but also minimize bleeding and postoperative complications. Laparoscopic surgery provides a high-definition, magnified, clear view, and recent advances in 3-dimensional laparoscopy and near-infrared cameras have enhanced the efficiencies of surgeries, such as LLND, that need a multidirectional approach.
A single-arm study reported the technical feasibility of laparoscopic LLND. In 2011, Konishi et al. [33] treated 14 patients laparoscopically, and the median amount of bleeding and operative time were 25 mL (range, 5–1,190 mL) and 413 minutes (range, 277–596 minutes), respectively. In the similar period, 16 patients underwent laparoscopic LLLND in Korea without conversion, and the mean blood loss was only 188 mL [34]. Furuhata et al. [35] reported the technical and oncologic safety of laparoscopic LLND without open conversion; no recurrences were detected during the mean 24-month follow-up.
Studies comparing laparoscopic versus open TME with LLND have reported laparoscopic surgery to be feasible and to have benefits such as lower blood loss and shorter hospital stay over open LLND. Ogura et al. [36] analyzed data from 107 patients and found the rates of major complications to be similar between the 2 groups (9.3% vs. 5.5%, P = 0.188). In another study with patient matching, laparoscopic surgery resulted in less blood loss (193 mL vs. 722 mL), similar postoperative complication rates (35.8% vs. 43.6%), and long-term survival [37]. A third study also reported similar results, including less blood loss, shorter hospital stays, and similar complication and survival rates [38].
In case of local recurrence after TME, adhesions make surgery a technically demanding procedure, but in this case, laparoscopic salvage LLND showed acceptable complications [39]. Therefore, although evidence for the feasibility and safety of laparoscopic LLND is limited, that procedure should be considered to be safe and oncologically feasible; in addition, it may have some advantages for short-term outcomes.
Robotic surgery for LLND was also feasible in terms of short-term and long-term oncologic outcomes. In 50 cases, Kagawa et al. [40] demonstrated that robotic procedure resulted in minimal blood loss and no conversion to open or laparoscopic surgery. Yamaguchi [41] reported that the overall survival and local relapse-free survival rate was similar between open and robotic LLND. Other small reports showed the feasibility of robotic LLND [42-44].
CONCLUSIONS
Over the past 30 years, the sexual and urinary functional outcomes after a LLND have improved due to autonomic nerve preservation and to advances in imaging modalities, such as high-resolution MRI, that enable more selective application of a LLND. Even with preoperative CRT, local pelvic recurrence is not preventable in about 5% to 10% of patients; nevertheless, a LLND may further decrease the local recurrence rate in patients with enlarged pelvic lymph nodes. With future study, we may be able to narrow the indications, in combination with the size of the lateral LN and the response to preoperative CRT, and minimize overtreatment. In addition, we need further study on the short-term advantages of using minimally invasive surgery for a LLND.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by a grant from National Cancer Center (grant number: 1810181-1).
