A Review of Bowel Preparation Before Colorectal Surgery
Article information
Abstract
Infectious complications are the biggest problem during bowel surgery, and one of the approaches to minimize them is the bowel cleaning method. It was expected that bowel cleaning could facilitate bowel manipulation as well as prevent infectious complications and further reduce anastomotic leakage. In the past, with the development of antibiotics, bowel cleaning and oral antibiotics (OA) were used together. However, with the success of emergency surgery and Enhanced Recovery After Surgery, bowel cleaning was not routinely performed. Consequently, bowel cleaning using OA was gradually no longer used. Recently, there have been reports that only bowel cleaning is not helpful in reducing infectious complications such as surgical site infection (SSI) compared to OA and bowel cleaning. Accordingly, in order to reduce SSI, guidelines are changing the trend of only intestinal cleaning. However, a consistent regimen has not yet been established, and there is still controversy depending on the location of the lesion and the surgical method. Moreover, complications such as Clostridium difficile infection have not been clearly analyzed. In the present review, we considered the overall bowel preparation trends and identified the areas that require further research.
INTRODUCTION
Infectious complications are a problem with bowel surgery, and one of the techniques that is constantly being studied to reduce them is bowel preparation. Preoperative bowel preparation traditionally refers to the removal of intestinal contents through mechanical cleaning with oral or rectal measures. The proposed benefits of using bowel preparations include reduced surgical site infection (SSI) rates, easier bowel manipulation during surgery, and reduced anastomotic leakage rates [1].
Since the 1980s, the successful results of emergency surgery without mechanical bowel preparation (MBP) have been linked to Enhanced Recovery After Surgery (ERAS). It has led surgeons to believe that MBP can be omitted. There have been many studies on the necessity of MBP. The conclusions of these studies were that MBP itself did not provide any benefit with regards to infection after colon and rectal surgery. Therefore, the trend of surgery gradually flowed in the direction of not performing MBP due to ERAS [2-4]. Following this trend, MBP using oral antibiotics (OA) also decreased.
However, the importance of OA has not disappeared. An analysis of a nationwide database in the United States in the 2010s revealed that the use of OA with MBP reduced SSIs compared to MBP alone [5-7]. As the use of OA for the prevention of infection after colon and rectal surgery has been re-examined, several meta-analyses have shown that the use of OA with MBP reduces postoperative infection [8-10]. There have been recent changes in the clinical guidelines in various countries, and the combination of MBP and OA is recommended [11-13].
HISTORY OF BOWEL PREPARATION
Since the 1930s, infection and leakage from the anastomosis have been important risk factors in colorectal surgery; therefore, MBP was performed without clear evidence that it actually reduced complications [14]. Following the discovery of antibiotics, Poth [15, 16] conducted many experimental and clinical studies using poorly absorbed OA to reduce the concentration of bacteria in the lumen during or after mechanical preparation. MBP was required before the use of effective intestinal antimicrobial agents. It reduced the burden of intraluminal bacteria and supported the antimicrobial action of OA on the mucosal surface [15, 17].
Starting with sulfathalidine [18], various antibiotic therapies have been studied, such as neomycin alone or a combination of neomycin and tetracycline. However, tetracycline was prohibited due to the occurrence of resistance [19]. Instead of these drugs, the combination of kanamycin and MBP was recommended to prevent SSI [20].
Antibiotic therapy has unquestionably had a profound effect on the practice of surgery, but it created a widespread superstition among surgeons that antibiotics could prevent all infections. This led to the indiscriminate use of antibiotics for prevention in all patients, which raised concerns regarding some serious problems that abusing antibiotics could cause [21]. Moreover, the studies on antibiotics at that time were not prospective or randomized clinical studies, and the level of evidence in the studies was low. These studies focused only on determining the microbiological effects of each drug [22].
In 1972, Nichols et al. [23] introduced a protocol using neomycin and erythromycin with MBP, which reduced the SSI rate from 43% to 9% [24, 25]. This protocol proved that the combination of preoperative OA and MBP could synergistically decrease the intestinal bacterial load prior to surgery, thus reducing the contamination of the operative field [12, 26]. Multiple trials from the 1970s to the 1990s demonstrated the effectiveness of this approach. However, it was found that systemic antibiotics (SA) with MBP were more effective than OA with MBP [26].
However, in the 1990s, as economic pressure in the United States reduced preoperative hospitalization, quicker MBP became the order of the day. MBP was poor and required large volumes of polyethylene glycol solution to achieve the gastrointestinal motility effects of oral erythromycin, especially in the elderly. Due to poor compliance and poor preparation, antibiotics were not effectively delivered throughout the bowel. The benefits of OA and MBP disappeared. Therefore, there was an opinion that only systemic prophylactic antibiotics could be used instead of OA with MBP [14].
Several clinical trials were conducted to find the optimal SA. The results of these studies showed that the SSI rate did not decrease in the MBP group compared to the non-MBP group [27-35]. Similarly, the results of meta-analyses demonstrated no benefit from MBP [36]. Therefore, MBP with antibiotics was largely abandoned because of concerns about the efficacy and safety of MBP [26].
Recently, there has been a general trend of reintroducing OA into preoperative MBP. Several surgical units have reported decreased SSIs after performing MBP combined with OA [37-39].
To better assess surgeon and hospital performance, the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) provides risk-adjusted results and models for comparison with goals that identify areas for improvement [40, 41]. The ERAS Society has produced colorectal-related bundles to further standardize healthcare services based on evidence-based practices [40, 42, 43]. Many organizations have implemented various infection prevention bundles to reduce SSI, and numerous projects have been implemented nationwide to reduce preventable complications [38, 44-49]. Although these infection prevention measures have been emphasized, the protocol is inconsistent and the role and use of OA and MBP are still diverse.
BOWEL PREPARATION IN THE PRESENT TIME
Mechanical bowel preparation vs. non-mechanical bowel preparation
MBP for colon surgery aims to reduce stool mass and bacterial count to decrease the rate of SSI. Most studies reported no difference in infectious complications with and without MBP.
After Hughes [50] first questioned the effectiveness of MBP before colorectal surgery, several reports have emerged regarding the potential benefits of MBP. They have not consistently identified significant reduction or prevention of various infectious complications such as SSI, anastomotic leakage, and abdominal abscess [3, 36, 51]. Furthermore, according to some studies, MBP was not recommended during bowel surgery due to various side effects and discomfort in patients. There are not many reports on complications of MBP and patient acceptability, but individual studies have cited abdominal discomfort, bloating, fatigue, dehydration, nausea, and preoperative complications, as well as abnormal electrolyte imbalances and risk of perforation, especially in the elderly [32, 52-56]. In addition, relatively safe results have been reported in cases where MBP was not performed, such as in an emergency. Since then, colorectal surgery has been attempted without MBP before surgery [27, 36, 52, 56]. Zmora et al. [56] reported that intraluminal liquid content was a common finding in the MBP group and that intestinal content leakage occurred more frequently (liquid content: 37.4% vs. 13.5%, P=0.0001; Spillage of content: 16.6% vs. 9.3%, P=0.046). However, the postoperative infection rate did not differ between the 2 groups.
Bucher et al. [34] reported an increased risk of morbidity with MBP for selective left-sided colorectal surgery, and Santos et al. [57] reported an increased risk of wound infection due to MBP compared with no preparation (24% vs. 12%), but no difference in the risk of anastomotic leakage.
Jung et al. [29] compared the MBP and non-MBP groups in 21 multicenter randomized clinical studies. In the 2 groups, the incidence of infectious complications was 7.9% and 6.8%, the incidence of SSI was 15.1% and 16.1%, and the anastomotic leakage rates were 2.3% and 2.6%, respectively; and there was no statistical difference. Therefore, it was concluded that MBP was no longer necessary as it was judged not to reduce the incidence of complications in colorectal surgery. Similar results have been reported in other studies [27, 58].
Slim et al. [4] reported that non-MBP did not have a negative effect on SSI compared to MBP in a meta-analysis; however, anastomotic leakage was more common in patients who underwent MBP than in patients who did not (5.6% vs. 3.2%; odds ratio [OR], 1.74; 95% confidence interval [CI], 1.05–2.90; P=0.032). This suggests that leakage of bowel contents following inappropriate MBP is a risk factor for infectious complications. The incidence of infectious complications such as SSI and anastomotic leakage is rather high in patients undergoing MBP for the following reasons. First, MBP causes local changes in the large intestine, which prevent wound healing. And second, changes in fluid and electrolytes caused by intestinal cleaning affect wound healing [3].
The results of randomized control trials comparing MBP and non-MBP in colon surgery show that there is no benefit of MBP in all aspects of SSI and anastomotic leakage [37, 59]. The results of several studies on MBP are summarized in Table 1.
Until 2011, systematic reviews such as the Cochrane Review collected and published various data after review and meta-analysis, and there were continuous updates, and eventually, the implementation of MBP showed that there was no further advantage [3,52, 59]. Therefore, several guidelines recommend that the implementation of MBP alone should be avoided to reduce SSI.
Mechanical bowel preparation + oral antibiotics effect
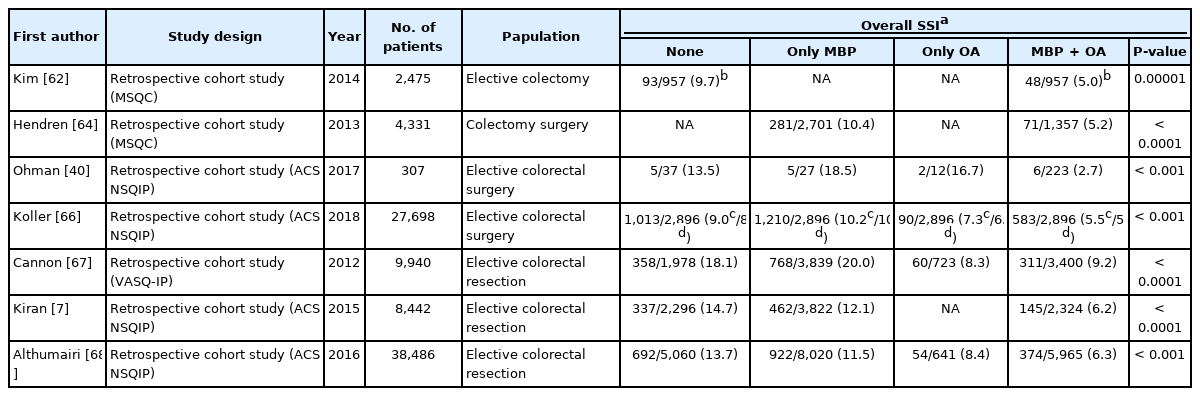
In a 1970s study comparing the effects of MBP and OA, it was reported that the SSI ratio in the MBP+OA group was smaller compared to that in the MBP alone group, but there was no confidence in the outcome [23]. However, in the early 1980s, it was reported that in the case of pretreatment using MBP and OA, postoperative complications could be further reduced from 20% to 7% [60, 61]. After these studies, the comparison between MBP and non-MBP in colorectal surgery was conducted by randomized and meta-analysis studies [52]. Table 2 summarizes the results of several studies on the effect of MBP+OA.
The Michigan Surgical Quality Collaborative made several reports, which consistently demonstrated the benefits of MBP+OA in the reduction of SSIs [62-64]. They included OA bowel preparations as 1 of the 6 elements of the colon surgery bundle to prevent SSI [65].
Ohman et al. [40] compared the MBP+OA combined group with the MBP alone group in the analysis of the NSQIP and reported that patients who received MBP+OA had an SSI rate of 2.7% compared with 15.8% for all others (P< 0.001). MBP+OA was reported to be the strongest independent factor (adjusted OR, 0.2; 95% CI, 0.1–0.9; P=0.006) influencing the reduction of SSI.
Koller et al. [66] classified patients into 4 groups (MBP, non-MBP, OA alone, and MBP+OA) in a retrospective study using the ACS NSQIP database of 32,359 people from 2012 to 2014, and compared the groups. The results were as follows; MBP was not associated with a reduction in the risk of SSI compared to the non-MBP group. Therefore, the use of MBP alone before regular colon surgery is not effective in preventing SSI and is no longer recommended. In contrast, OA were associated with a reduced risk of all types of SSIs. The MBP+OA group had a significantly reduced risk of SSI and there was no increased risk of other complications compared to the MBP and non-MBP groups. In the multivariate analysis, the OR of the SSI frequency, anastomotic leakage rate, and mortality rate of MBP+OA ranged between 0.43 and 0.57, which is a very important result [52]. According to the study, although prospective studies are needed to determine the efficacy and risk of OA before colon surgery, MBP and OA should be used in combination before elective colon resection.
In a recent large-scale study in the United States, the combined use of MBP and OA was an independent factor in reducing the incidence of anastomotic leakage, SSI, and postoperative paralytic ileus when compared with the OA or MBP alone [7, 67, 68]. As a result, in 2019, in the American Society of Colon and Rectal Surgeons Clinical Practice guidelines [37], MBP combined with preoperative OA is typically recommended for elective colorectal resections (Grade of Recommendation: Strong recommendation based on moderate-quality evidence, 1B).
MBP+OA was reflected in the World Health Organization guidelines. In addition, the ACS and Surgical Infections Society guidelines also recommend the combined use of MBP and OA. The recommended regimen of OA is not clear, but nonabsorbable antibiotics covering gram-negative and aerobic bacteria should be administered [12].
Only oral antibiotics
OA alone has been reported to reduce SSI [67]; however, it is not sufficient to improve all postoperative outcomes compared to MBP+OA.
In a 2016 randomized study comparing OA and OA+SA before colon surgery, Hata et al. [69] found that SSI decreased in patients receiving OA +SA rather than SA alone (7.3% vs. 12.8%; P=0.028). Until clear research results are obtained, the combination of MBP and OA should be considered, rather than only OA.
CURRENT ISSUES OF BOWEL PREPARATION
Rectal surgery
In the case of anastomosis after rectal resection, there may be differences in the interpretation of the results because various methods such as MBP, non-MBP, and simple enema are used for preoperative bowel cleaning. Tables 3, 4 summarize the results of rectal surgery.
Bretagnol et al. [70] reported specifically on elective sphincter-preserving surgery for rectal cancer from the French Research Group of Rectal Cancer Surgery (GRECCAR) III randomized controlled trial. They demonstrated that rectal surgery without preoperative MBP was significantly associated with an increase in the 30-day overall morbidity and infectious complication rates (non-MBP vs. MBP: 44% vs. 27%, P = 0.018 and 34% vs. 16%, P=0.005, respectively). In addition, there was a trend toward an increased risk of anastomotic leakage (19% vs. 10%, P=0.09) and peritonitis (7% vs. 2%, P=0.15), but the differences were not statistically significant [70].
Golder et al. [71] said that the use of preoperative OA+MBP+intravenous (IV) antibiotics for specific left bowel or rectal surgery can effectively reduce postoperative systemic inflammatory response and postoperative complications: overall complications (OR, 0.31; 95% CI, 0.17–0.56; P< 0.001), infective complications (OR, 0.41; 95% CI, 0.22–0.77; P=0.011), and SSI (OR, 0.37; 95% CI, 0.17–0.83; P=0.024).
Ghuman et al. [26] reported a significant reduction in overall SSI rates (26.2% vs. 15.3%, P = 0.02) and superficial SSI rates (11.1% vs. 4.4%, P=0.02), but not in organ space SSI rates (15.1% vs. 10.9%, P=0.18).
Zmora et al. [35] reported no difference in the rate of surgical infectious complications between the non-MBP and MBP groups. (13.2% vs. 12.5%). Similarly, Van’t Sant et al. [33] reported that there was no significant difference between the MBP group and the non-MBP group in the occurrence of anastomotic leakage or septic complications (7.6% vs. 6.6%). On the other hand, Bucher et al. [34] reported that the infectious complication rate was higher in the MBP group (22% vs. 8%, P=0.028).
According to the Cochrane Review [59], there was no difference in the results of anastomotic leakage and SSI rates between MBP and non-MBP patients after low anterior resection. However, other studies have shown that the overall prevalence of infection was higher in the non-MBP group after elective surgery for rectal cancer [51, 52, 71]. There are very few randomized comparative studies between the MBP and non-MBP strategies related to rectal surgery.
In low rectal surgery, there is a variable called temporary diverting ileostomy, which is believed to reduce the incidence of anastomotic leakage, but this is still controversial [33]. Further research on MBP or enemas versus no preparation in patients undergoing elective rectal surgery is needed.
Minimally invasive surgery
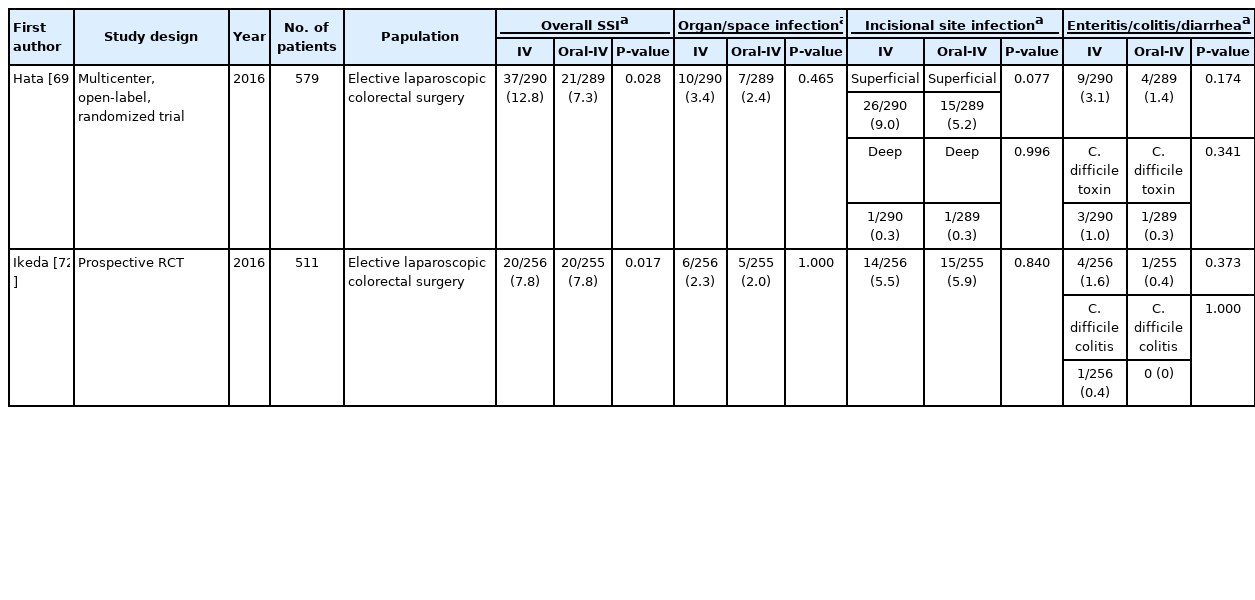
Recently, laparoscopic surgery has become a primary surgical option for patients with colorectal cancer because of its several advantages over open surgery. There have been many randomized clinical trials on the benefits of MBP, but they are still unclear, especially for laparoscopic colorectal surgery [36]. In practice, the distended intestine interferes with the visual field during laparoscopic surgery and can make intestinal manipulation more difficult, so it is necessary to consider MBP [4].
Hata et al. [69] performed a multicenter randomized controlled trial to confirm the efficacy and regimen of oral and parenteral antibiotic prophylaxis in elective laparoscopic colon surgery. The incidence of SSIs in laparoscopic colorectal surgery remains high, at around 8% to 23%. Although several guidelines have been published for antibiotic prophylaxis in cases of colorectal surgery, no previous studies have determined the optimal antibiotic regimen for laparoscopic colorectal surgery. Oral and parenteral prophylaxis (oral, kanamycin and metronidazole; IV, cefmetazole) significantly reduced the incidence of SSIs in patients undergoing elective laparoscopic colorectal surgery without significantly increasing the incidence of enteritis/colitis/diarrhea and Clostridium difficile toxin in stool samples (7.26% vs. 12.8%; OR, 0.536; 95% CI, 0.305–0.940; P=0.028). Ikeda et al. [72] argued that with regard to SSI in patients with colorectal cancer undergoing elective laparoscopic resection, IV perioperative antimicrobial prophylaxis alone is not inferior to combined preoperative oral and IV prophylaxis (overall: 7.8% vs. 7.8%, P=0.017; incisional site: 5.5% vs. 5.9%; organ/space infection: 2.3% vs. 2.0%) (Table 5). Therefore, it is necessary to confirm whether MBP+OA +IV prophylaxis should be performed even in minimally invasive surgeries such as laparoscopic surgery.
Clostridium difficile infection
One of the concerns about the use of OA in patients undergoing colorectal surgery is C. difficile infection. Al-Mazrou et al. [73] conducted a study using ACS NSQIP data (2015 and 2016) on the incidence of OA-induced C. difficile infection in patients undergoing colon resection. They reported a 1% to 7% incidence of C. difficile infection after surgery; in the multivariate analysis, in the group that underwent preoperative OA and MBP, OA significantly lowered postoperative C. difficile infection (OR,0.6; 95% CI,0.5–0.8). In patients without postoperative infectious complications, the use of OA has been reported to lower the incidence of C. difficile infection in all subgroups (OR,0.7; 95% CI,0.5–0.9) [52, 73].
Kim et al. [62] collected data from the Michigan Surgical Quality Collaborative-Colectomy Best Practices Project and analyzed them by dividing them into groups of full bowel preparation (MBP with nonabsorbable OA) or no bowel preparation (neither MBP nor nonabsorbable OA administered). They report that patients receiving full preparation were less likely to develop postoperative C. difficile colitis (0.5% vs. 1.8%, P=0.01).
Wren et al. [74] reported that the rate of postoperative C. difficile colitis was 4.2% in the entire study population. In this study, the rate of C. difficile infection was higher in patients who received OA than in those who did not (7.4% vs. 2.6%, P=0.03). The use of nonabsorbable OA in bowel preparation resulted in a higher rate of C. difficile infection.
The risk of C. difficile infection as a complication of OA bowel preparation is still not consistent.
Antibiotic regimen
Prophylactic antibiotics are needed to prevent infectious complications. However, uncontrolled analysis of the various routes, methods, and durations of administration remains problematic. Among the administration methods, only a few studies have been conducted on IV antibiotics, and preoperative intestinal cleanin methods are also diverse. The types and frequency of drugs used as prophylactic antibiotics, whether oral or IV, are also inconsistent. Therefore, it is difficult to draw a consistent conclusion [15].
Studies in the 1970s showed that oral kanamycin+erythromycin or neomycin+erythromycin was very effective in removing many intestinal pathogens associated with infection after surgery [75-79]. Recently, as the necessity of bowel cleanliness has emerged again, studies using various antibiotic regimens have been conducted. Most studies used a combination of either ceftriaxone, cefoxitin, cefuroxime, flomoxef, cephazolin, amikacin with or without metronidazole with or without gentamicin, or ticarcillin-clavulanate as the IV antibiotics of choice [80]. The most common OA therapy is neomycin+metronidazole, followed by kanamycin or neomycin with metronidazole, but microbiological evidence has not yet been established [80, 81]. In some studies, gentamicin+metronidazole was administered as routine prophylaxis for 24 hours, 48 hours, or longer after surgery [8, 82]. Other studies included oral sulfamethoxazole/trimethoprim+metronidazole or doxycycline+metronidazole or oral neomycin+metronidazole within 24 hours before surgery, and IV administration ceftriaxone+metronidazole at induction [8, 31, 52].
While many other poorly absorbed antibiotics are available, comparative studies of alternative drugs have been conducted in the last 20 years. However, the results of the analysis and evaluation of the timing of completion of OA prior to surgical intervention were not clearly obtained, and further studies are needed [14]. Therefore, the search for better mechanical and antibiotic preparation strategies should be reactivated.
CONCLUSION
To date, MBP has no effect on reducing SSI when used alone. It has been found to be effective only when used with OA. Therefore, various guidelines have been changed and MBP+OA recommended. However, the results of the analysis of the surgical method, surgical site, and side effects of antibiotic use are not yet consistent, and the ideal antibiotic regimen has not yet been confirmed. Therefore, more research will have to be conducted to specify a clear method of cleaning the intestines.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.