Ileal Pouch-Anal Anastomosis for Ulcerative Colitis: An Australian Institution’s Experience
Article information
Abstract
Purpose
We report outcomes and evaluate patient factors and the impact of surgical evolution on outcomes in consecutive ulcerative colitis patients who had restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) at an Australian institution over 26 years.
Methods
Data including clinical characteristics, preoperative medical therapy, and surgical outcomes were collected. We divided eligible patients into 3 period arms (period 1, 1990 to 1999; period 2, 2000 to 2009; period 3, 2010 to 2016). Outcomes of interest were IPAA leak and pouch failure.
Results
A total of 212 patients were included. Median follow-up was 50 (interquartile range, 17 to 120) months. Rates of early and late complications were 34.9% and 52.0%, respectively. Early complications included wound infection (9.4%), pelvic sepsis (8.0%), and small bowel obstruction (6.6%) while late complications included small bowel obstruction (18.9%), anal stenosis (16.8%), and pouch fistula (13.3%). Overall, IPAA leak rate was 6.1% and pouch failure rate was 4.8%. Eighty-three patients (42.3%) experienced pouchitis. Over time, we observed an increase in patient exposure to thiopurine (P = 0.0025), cyclosporin (P = 0.0002), and anti-tumor necrosis factor (P < 0.00001) coupled with a shift to laparoscopic technique (P < 0.00001), stapled IPAA (P < 0.00001), J pouch configuration (P < 0.00001), a modified 2-stage procedure (P = 0.00012), and a decline in defunctioning ileostomy rate at time of IPAA (P = 0.00002). Apart from pouchitis, there was no significant difference in surgical and chronic inflammatory pouch outcomes with time.
Conclusion
Despite greater patient exposure to immunomodulatory and biologic therapy before surgery coupled with a significant change in surgical techniques, surgical and chronic inflammatory pouch outcome rates have remained stable.
INTRODUCTION
Ulcerative colitis (UC), one of 2 major forms of inflammatory bowel disease (IBD), is a chronic remittent or progressive inflammatory condition affecting the colonic mucosa [1]. Up to a quarter of UC patients will need colectomy in their lifetime despite medical therapy [2, 3].
Restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) is considered the preferred option for managing treatment-refractory severe UC when the patient wishes to avoid a permanent stoma [4]. Since it was first described by Parks and Nicholls in 1978 [5], the IPAA has undergone a number of technical modifications. However, complication rates associated with IPAA have been reported to be up to 50% [6] and the long-term risk of pouch failure is 0.9% to 13% [7-11].
The primary objective of this study was to evaluate factors associated with perioperative complications and long-term pouch failure. The secondary objective was to evaluate patient factors and the impact of surgical evolution on outcomes for patients who had IPAA for UC over a period of 26 years [12, 13].
METHODS
All consecutive UC patients who underwent IPAA between February 1990 to August 2016 at the Royal Brisbane and Woman’s Hospital (RBWH) in Brisbane, Australia were included. The RBWH provides secondary and tertiary IBD and colorectal surgical care to patients using the public healthcare service across north Brisbane and to regional hospitals north of the city across an area of approximately 375,000 km2. Patients who had IPAA for familial adenomatous polyposis (FAP), revision and recreation of existing ileal-anal pouches were excluded. Ethics approval for this research was obtained through the ethics committee of Royal Brisbane and Women’s Hospital (No. 14/QRBW/323). Written informed consent was waived.
This is a descriptive study with both prospective and retrospective data collection through the IBD software program (IBD Prime), a password protected and encrypted database. To ensure comprehensive patient capture, names were cross-referenced with colorectal surgery databases to identify patients who had IPAA at RBWH. For each patient, demographic details and clinical characteristics preoperative, operative, and postoperative details were documented. A physician (MHL) reviewed all patient records to supplement the existing prospectively collected data.
Patients were divided into 3-period arms based on the year of IPAA surgery (period 1, 1990 to 1999; period 2, 2000 to 2009; and period 3, 2010 to 2016).
Definitions
One-stage procedure: proctocolectomy with IPAA without protective ileostomy. Traditional 2-stage procedure: proctocolectomy with IPAA and loop ileostomy followed by ileostomy closure. Modified 2-stage procedure: subtotal colectomy with end ileostomy followed by proctectomy with IPAA without protective ileostomy. Three-stage procedure: subtotal colectomy with end ileostomy followed by proctectomy with IPAA with protective ileostomy followed by ileostomy closure. Early complications: complications within 30 days of IPAA or closure of ileostomy for patients with a loop ileostomy formed at the time of IPAA [8]. Late complications: complications > 30 days after IPAA or closure of ileostomy for patients with a loop ileostomy formed at the time of IPAA. IPAA leak: a defect in IPAA found at endoscopy, operation, or other imaging studies within 3 months of IPAA construction. Anal stenosis: IPAA narrowing necessitating dilatation. Pelvic sepsis: pelvic abscess, anastomotic leakage, or dehiscence. Small bowel obstruction (SBO): discharge diagnosis of patient recorded as SBO and/or diagnosis of SBO confirmed on imaging studies and/or at surgery. Pouch fistula: any pouch-related fistula. Pouch failure: permanent diversion and/or pouch excision. Acute pouchitis: combination of clinical presentation and endoscopic findings (hyperaemic and/or hemorrhagic friable and granular mucosa with excessive mucopurulent areas and superficial erosions) [10].
The definitions for chronic pouchitis and Crohn disease-like (CDL) phenotype were those described by Tyler et al. [14].
Statistical analysis
Statistical analysis was performed on de-identified data only. Patient characteristics and clinical data were expressed as median (interquartile range [IQR]). Quantitative variables between groups were compared using Kruskal-Wallis test while categorical variables were compared using a one-way analysis of variance as appropriate. Odds ratios (OR) and 95% confidence intervals (CI) were estimated using univariate logistic regression analysis to quantify the association between the outcome and possible factors. Variables with a P-value of < 0.05 in the univariate analysis were selected for multivariate analysis. Statistical significance was set at P < 0.05.
RESULTS
Patient demographics and clinical characteristics
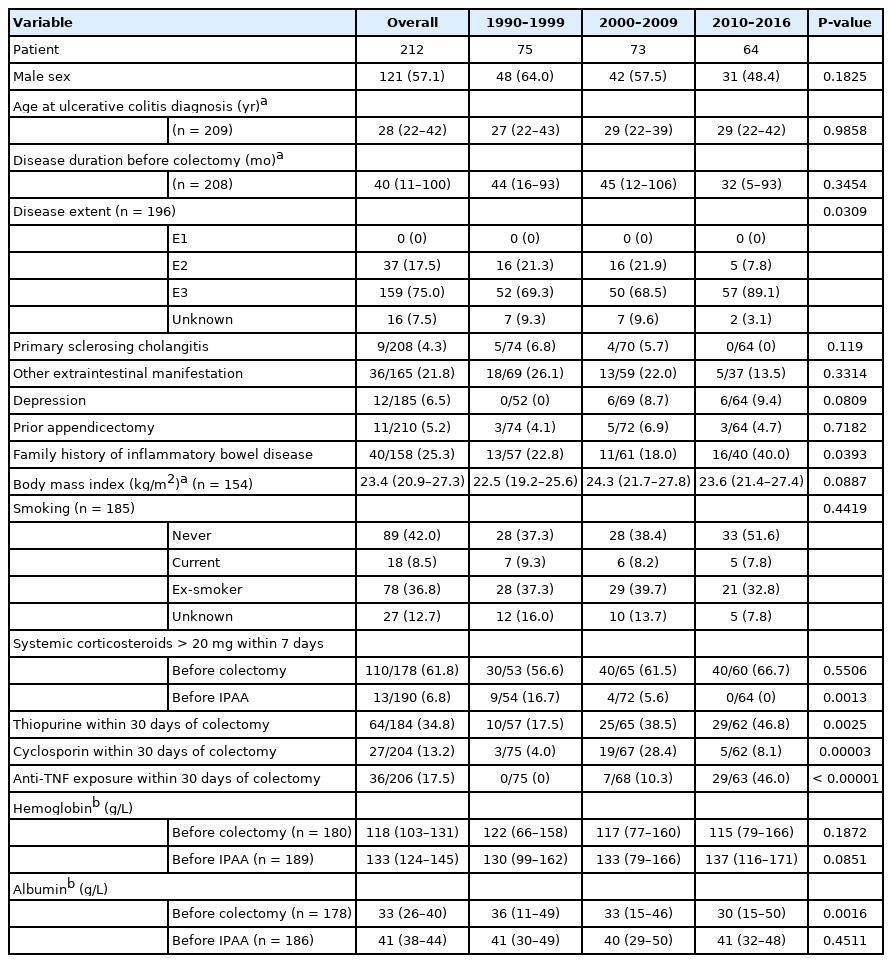
Overall, 212 patients (57.1% male) were included. The majority had extensive disease and had received high-dose corticosteroids within 7 days of colectomy (Table 1). The median preoperative disease duration was 40 months (IQR, 11 to 100 months). Nine patients (4.3%) had primary sclerosing cholangitis and 11 patients (5.2%) had a prior appendicectomy. Patients receiving thiopurine (34.8%), cyclosporin (13.2%), and/or anti-tumor necrosis factor (TNF) agents (17.5%) before colectomy were the minority.
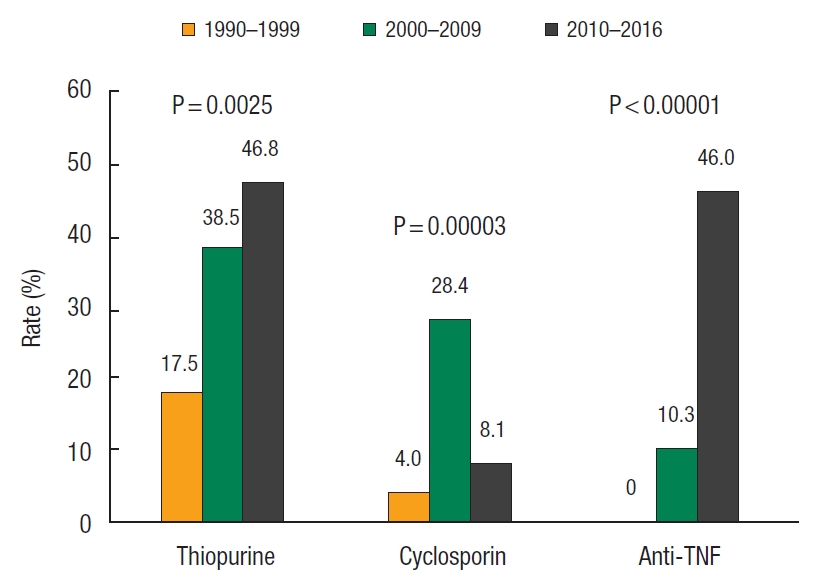
Over the different periods, fewer patients were on > 20 mg/day corticosteroids before IPAA (period 1, 16.7%; period 2, 5.6%; period 3, 0%; P = 0.0013). An increase in patient exposure to thiopurine (period 1, 17.5%; period 2, 38.5%; period 3, 46.8%; P = 0.0025) and anti-TNF agents (period 1, 0%; period 2, 10.3%; period 3, 46.0%; P < 0.00001) were observed. The use of cyclosporin increased initially but decreased in recent years (period 1, 4.0%; period 2, 28.4%; period 3, 8.1%; P = 0.00003) (Fig. 1).
Operative details
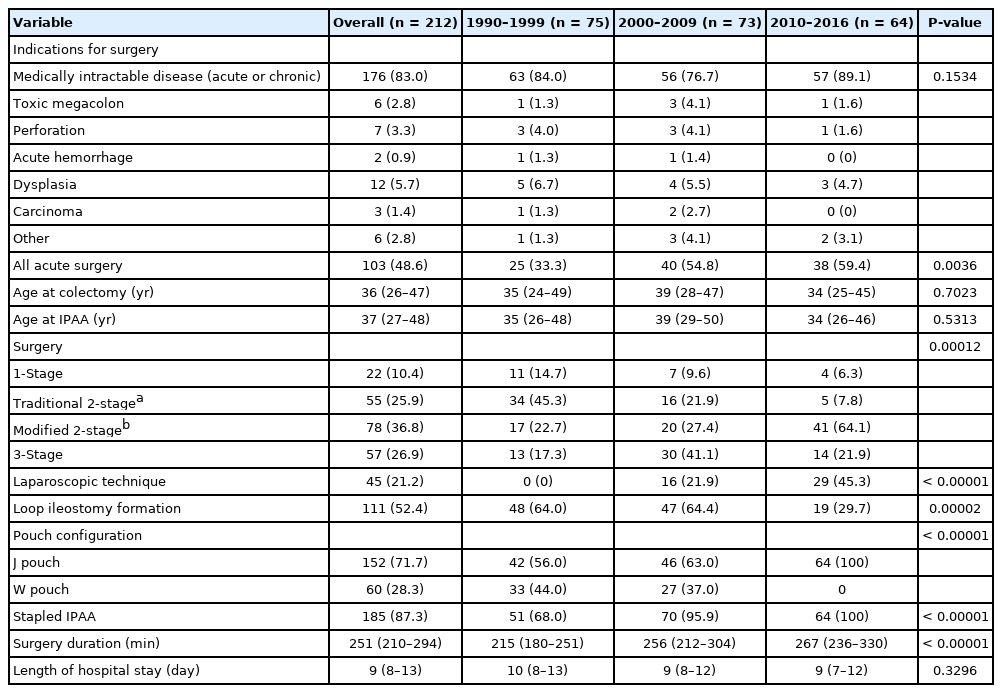
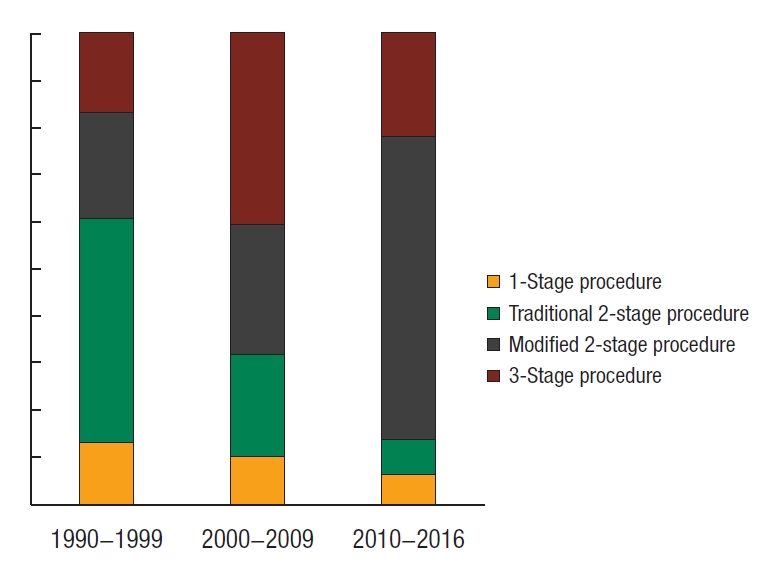
Twenty-two patients (10.4%) had a one-stage procedure, 55 patients (25.9%) had a traditional 2-stage procedure, 78 patients (36.8%) had a modified 2-stage procedure, and 57 patients (26.9%) had a 3-stage procedure (Table 2). A total of 109 patients (51.4%) had surgery for chronic indications. For the 103 patients who had surgery for acute indications (acute surgery), the modified 2-stage procedure (n = 57, 55.3%) and the 3-stage procedure (n = 45, 43.7%) were the predominant procedures. The median age at the time of IPAA was 37 years (IQR, 27 to 48 years). Forty-five IPAA (21.2%) were performed laparoscopically. The median length of stay after IPAA was 9 days (IQR, 8 to 13 days). Median follow-up was 50 months (IQR, 17 to 120 months).
Across the different periods, more patients were having acute surgery (period 1, 33.3%; period 2, 54.8%; period 3, 59.4%; P = 0.0036) with the 3-stage procedure favored in period 2 (period 1, 36.0%; period 2, 60.0%; period 3, 31.6%; P = 0.0268). We observed a shift to laparoscopic technique (period 1, 0%; period 2, 21.9%; period 3, 45.3%; P < 0.00001), stapled IPAA (period 1, 68.0%; period 2, 95.9%; period 3, 100%; P < 0.00001), J pouch configuration (period 1, 56.0%; period 2, 63.0%; period 3, 100%; P < 0.00001), a modified 2-stage procedure (period 1, 22.7%; period 2, 27.4%; period 3, 64.1%; P = 0.00012) (Fig. 2) and a decline in defunctioning ileostomy use at time of IPAA (period 1, 64.0%; period 2, 64.4%; period 3, 29.7%; P = 0.00002). Median surgery duration increased (period 1, 215 minutes; period 2, 256 minutes; period 3, 267 minutes; P < 0.00001). Median length of hospital stay after IPAA was similar across periods 1 to 3 [12].
Post-IPAA complications
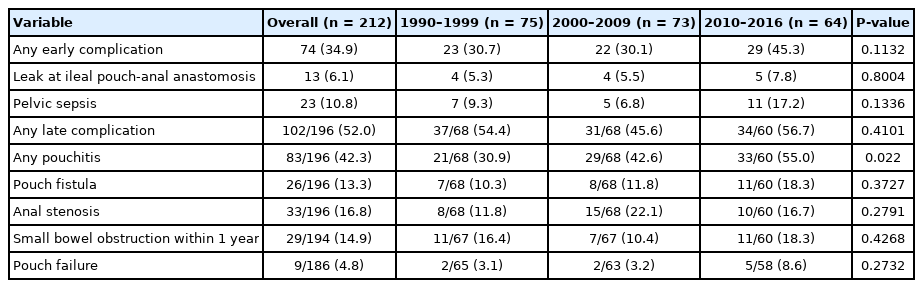
The rates of early and late complication were 34.9% and 52.0%, respectively (Table 3). Early complications included wound infection (n = 20, 9.4%), pelvic sepsis (n = 17, 8.0%), SBO (n = 14, 6.6%), and urinary tract infection (n = 7, 3.3%). Four patients had venous thromboembolism and 2 patients had portal vein thrombosis after IPAA. Late complications included SBO (n = 37, 18.9%), anal stenosis (n = 33, 16.8%) and pouch fistula (n = 25, 12.8%). Patients receiving a defunctioning ileostomy at the time of IPAA were more likely to have SBO as compared to those who did not (28.8% vs. 8.5%, P = 0.0414).
Overall, IPAA leak rate was 6.1% and pouch failure rate was 4.8%. Median time to pouch failure was 35 months (IQR, 22 to 102 months). Causes of pouch failure were pouch fistulae (n = 4), pouch dysfunction (n = 2), incontinence (n = 1), recurrent anal stenosis (n = 1), and refractory pouch bleeding (n = 1). The rates of SBO, anal stenosis, IPAA leak, and pouch failure over time were not significantly different [12].
There was 1 patient diagnosed with anal squamous cell carcinoma 257 months after IPAA and 9 deaths, all of which were not related to UC or IPAA [13].
Inflammatory pouch outcomes
Eighty-three patients (42.3%) had at least 1 episode of acute pouchitis. Chronic pouchitis and CDL phenotype occurred in 8.7% and 11.6% of patients, respectively. Acute pouchitis rates increased over time (period 1, 30.9%; period 2, 42.6%; period 3, 55.0%; P = 0.022). However, chronic pouchitis (period 1, 9.7%; period 2, 6.9%; period 3, 9.4%; P = 0.8423) and CDL phenotype (period 1, 8.1%; period 2, 15.5%; period 3, 11.3%; P = 0.4467) remained stable.
Risk factors for complications
Results for univariate and multivariate risk factors can be found in Table 4.

Univariate analysis for IPAA leak, pouch fistula, and pouch failure as well as multivariate model for pouch failure
Univariate risk factors
A body mass index (BMI) above 18.1 kg/m2 was protective against IPAA leak (OR, 0.15; 95% CI, 0.04 to 0.58; P = 0.002). Steroid use before colectomy (> 20 mg/day) was associated with IPAA leak (OR, 7.44; P = 0.028), pouch fistula (OR, 3.7; P = 0.016), and pouch failure (OR, infinity; P = 0.03) on univariate analysis. Steroid use before pouch surgery (>20 mg/day) was also associated with pouch failure.
Multivariate risk factors
• Anastomotic leak: multivariate analysis revealed a BMI above 18.1 kg/m2 before surgery was protective (OR, 0.81; 95% CI, 0.74 to 0.88; P = 0.002) while steroid use (> 20 mg/day) before colectomy was a risk factor at the trend level (OR, 1.09; 95% CI, 1.00 to 1.18; P = 0.053).
• Pouch fistula: anastomotic leak (OR, 1.25; 95% CI, 1.03 to 1.52; P = 0.022), and acute surgery indication (OR, 1.12; 95% CI, 1.01 to 1.23; P = 0.028) were observed as risk factors on multivariate analysis while stapled IPAA was a risk factor at the trend level (OR, 1.14; 95% CI, 0.98 to 1.32; P = 0.083). Age ≥ 30 years at the time of surgery was observed to be a protective factor in the same model (OR, 0.88; 95% CI, 0.80 to 0.98; P = 0.019).
• Pouch failure: both IPAA leak (OR, 1.19; 95% CI, 1.06 to 1.33; P = 0.0041) and pouch fistula (OR, 1.20; 95% CI, 1.10 to 1.31; P < 0.0001) were observed as risk factors for pouch failure. No other factors were statistically significant. The association observed between steroid use before pouch surgery (> 20 mg/day) and pouch failure on univariate analysis was not observed after adjusting for potential confounders.
DISCUSSION
The objectives of this study were to review our institution’s experience with IPAA for UC patients, evaluate factors associated with perioperative complications and long-term pouch failure in our unit, and evaluate patient factors and the impact of medical therapy and surgical evolution on outcomes over a period of 26 years.
Preoperative steroid use in IBD patients undergoing abdominal surgery has been associated with an increased risk of total and infectious complications in a meta-analysis of 1,532 patients [15]. In a recent study on 758 UC patients who had stapled IPAA, preoperative steroid use (> 15 mg) was an independent risk factor for pouch leak (OR, 1.61) [9]. In our cohort, a trend toward IPAA leak was observed with preoperative steroid use (> 20 mg/day) on multivariate analysis. Over the eras and with the evolution to the modified 2-stage approach, no patients were on pre-IPAA steroids in the third decade. Obesity (BMI ≥30 kg/m2) was previously identified as an independent risk factor for pouch-related complications [16]. We observed an association between BMI ≤ 18.1 kg/m2 before surgery and an increased risk of IPAA leak. In our analysis, age< 30 years, an acute surgery indication, and IPAA leak were associated with an increased risk of pouch fistula. In a previous study, female sex, previous anal pathology especially perianal abscess or fistula in ano, a diagnosis of Crohn disease and pelvic sepsis were risk factors associated with pouch-related fistula [17]. Additionally, IPAA leak and pouch fistula were identified as independent risk factors for pouch failure in our cohort, consistent with published literature [7, 18, 19].
The majority of published outcomes following IPAA consist of a mixed cohort of patients including those with FAP. In the largest published single-institution series of IPAA in the literature, Fazio et al. [7] reported the outcomes of 3,707 patients including 2,953 (80%) with UC who have had IPAA at the Cleveland Clinic over a 26-year period and their reported rates of anastomotic leak, SBO, anal stricture, and pouch failure were 4.8%, 18.1%, 16.5%, and 5.1%, respectively. Our rates of IPAA leak (6.1%), SBO (14.9%), pouch fistula (13.3%), anal stenosis (16.8%), and pouch failure (4.8%) were not inferior. The 2017 Ileoanal Pouch Report [11] including voluntarily submitted data on 5,083 primary ileoanal pouch surgeries performed at 76 United Kingdom and 4 European centers reported a similar overall pouch failure rate (4.7%) but much lower rates of anastomotic leak (3.3%), SBO (5.3%), and pouch fistula (4.7%).
IPAA outcomes for UC patients alone were recently published by the Mount Sinai Hospital group in Toronto, Canada [9] and the Leuven group in Belgium [10]. The Canadian experience in 758 UC patients who had stapled IPAA over an 11-year period had a higher leak rate at 12.1% but much lower rates of pouch fistula (3.4%), anal stricture (7.3%), and pouch failure (0.9%) although the median duration of follow-up was not reported. The Belgian experience in 335 UC patients who had IPAA between 1990 and 2015 also reported a higher anastomotic leak rate at 14.9% but similar rates for SBO (12.2%), anastomotic stricture (14.3%), and pouch failure (5.9%).
In our institution and in the mid-2000s, there was a conscious shift to J pouch construction, from W pouches, which was supported by the randomized trial comparing the long-term functional outcomes of the J and W pouch undertaken by our colorectal surgeons. McCormick et al. [20] found that the functional benefit of W pouches at 1 year was of little consequence to patients’ long-term quality of life with no significant difference in functional outcomes between J and W pouches at 9 years. We also observed a shift to the modified 2-stage procedure, stapled anastomoses, increasing rate of laparoscopic surgery, and less use of a defunctioning ileostomy. This is consistent with the surgical evolution over time reported by the Leuven group [10] and the 2017 Ileoanal Pouch Report [11]. Additionally, the Leuven group observed a significant decrease in anastomotic leak and SBO rates with the surgical evolution.
The observed reduction in the use of diverting loop ileostomy corresponded with the shift toward the modified 2-stage procedure. In this scenario, patients are off all immunosuppression and are in a better nutritional state before they undergo their definitive pouch procedure. Defunctioning ileostomies are not without morbidity themselves. Readmission rates are high with a recent large retrospective review showing a 28% readmission rate within 60 days; most commonly for dehydration [21] or obstruction [22]. Fielding et al. [23] reported a 2.2-fold increase in rates of new or worsening chronic kidney disease compared with patients without an ileostomy and that these findings persist despite closure of the ileostomy. There is also the need for further surgery to close the ileostomy and its subsequent risk of incisional hernia. It was observed that the decrease in ileostomy rate did not translate into an increase in surgical complications or an increase in anastomotic leak rate.
We documented a significant increase in patient exposure to immunomodulatory and biologic therapy with time, and a decrease in corticosteroid use at the time of IPAA. Interestingly, these observed changes did not alter post-IPAA outcomes significantly in our cohort. We did, however, observe a trend toward increasing rates of early complications, pelvic sepsis, pouch fistula, and pouch failure with time. The reason for this observation is unclear. A plausible explanation would have been an increase in patient exposure to corticosteroids, immunomodulatory and/or biologic therapy before surgery. However, no patients (0%) were on > 20 mg corticosteroids before IPAA in period 3 compared to 6% in period 2 and 17% in period 1. Additionally, the proportion of patients who underwent a modified 2-stage procedure or a 3-stage procedure (period 1, 40.0%; period 2, 68.5%; period 3, 85.9%) would not have been on immunomodulatory and/or biologic therapy before IPAA as these would have been discontinued post colectomy. However, a delayed effect of these medications on immune function and the “pelvic microbiome” cannot be discounted [24, 25] given that the exposure to thiopurines, ciclosporin, and anti-TNF therapies, within 30 days of colectomy, all increased significantly together with the proportion of patients undergoing surgery for acute severe disease. In terms of patient nutritional status, pre-IPAA BMIs and pre-IPAA albumin levels were not significantly different across different periods whereas precolectomy albumin levels dropped significantly between period 1 (36 g/L; range, 11 to 49 g/L) and period 3 (30 g/L; range, 15 to 30 g/L) consistent with the above changes in treatment and indication.
The Leuven group [10] observed a significant decrease in urgent surgeries (defined as surgeries performed at the time of hospitalization to treat an intractable flare or acute severe colitis) and a corresponding increase in median duration between UC diagnosis and IPAA from 1990 to 2015. This was thought likely to be the result of improving medical options but data were not available to prove this hypothesis. In contrast, we observed a significant increase in IPAA for acute indications and no difference in median duration of disease before surgery from 1990 to 2016. This was despite a significant increase in immunomodulator use in this subgroup over time (period 1, 11.8%; period 2, 32.4%; period 3, 45.9%; P = 0.04645). These differences between centers may in part be related to differences in referral patterns.
Our overall rate of pouchitis (42.3%) was similar to the Leuven group (41.5%) [10] and in line with other large studies [7, 18]. However, we observed a significant increase in pouchitis rates over time while stable rates were reported by the Leuven group. This could be a reflection of UC patients needing surgery in recent years having the more aggressive disease as evidenced by more surgeries for acute indications, lower albumin levels before colectomy, and more patients with the extensive disease which in turn is a known risk factor for pouchitis [26]. Probiotics are not routinely offered to post-IPAA patients.
This study has some limitations. Firstly, this is a single-center retrospective study that is limited by relatively small numbers and relatively short follow-up. Secondly, information bias could be a factor if the notes for patients who had IPAA in period 3 were more complete compared to those who had IPAA in periods 1 and 2 given the retrospective nature of some of the data collection. Thirdly, post-IPAA functional aspects including fecundity, sexual function, and pouch function were not evaluated. Some of these limitations could be addressed by prospective data collection in the future using validated questionnaires.
In conclusion, compared with published IPAA complication rates from larger centers, IPAA for UC performed at our institution is safe. Surgical and chronic inflammatory pouch outcome rates have remained stable over time despite greater patient exposure to immunomodulatory and biologic therapy before surgery coupled with a shift toward more laparoscopic procedures, stapled anastomoses, modified 2-stage procedures, and reduced use of defunctioning ileostomy over time.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
Acknowledgements
Anton Lord and Lisa Simms were supported by philanthropic grants from the Roberts family and the McGuire family.