The “reverse air leak test”: a new technique for the assessment of low colorectal anastomosis
Article information
Abstract
Purpose
Anastomotic leakage is a fearsome complication in rectal surgery. Surgeons perform the classic air leak test, although its real effectiveness is still debated. The aim of this study was to describe a personal technique of reverse air leak test in which low colorectal anastomosis was assessed transanally through the intrarectal irrigation of a few mL of saline solution.
Methods
From October 2014 to November 2019, 11 patients with low rectal cancer (type 1 in Roullier classification) were included in this study. At the beginning of the procedure, a circular anal dilator was inserted into the anus. A side-to-end colorectal anastomosis was performed. A few mL of saline solution were injected into the rectum and the entire anastomotic line was directly explored. The appearance of bubbles was considered as an anastomotic defect and repaired with an interrupted suture. A fluorescence angiography after intravenous injection of indocyanine green was performed in order to evaluate the perfusion of the anastomosis.
Results
The reverse air leak test was positive in 4 cases (36.4%). The defect was repaired and a confirmation test was performed. In all patients, near-infrared evaluation showed no perfusion defect (grade 0) in low colorectal anastomosis. No postoperative fistula was detected in cohort study. A protective stoma was performed in 10 patients. On day 90, there were no complications and stoma closure was performed as planned.
Conclusion
The reverse air leak test is a simple, feasible, and effective procedure to identify anastomotic leaks in low colorectal anastomoses.
INTRODUCTION
Anastomotic leakage is the most frequent complication after anterior rectal resection with an estimated range from 3% to 28% [1-3]. It usually involves a variation of the normal postoperative course, a prolongation of the hospital stay, up to a surgical reoperation, and a significant increase in 90-day mortality of 3.9% in a recent Swedish population-based cohort study [3].
From a functional point of view, anastomotic fistula is associated with deterioration in the quality of life with an increased risk of fecal incontinence and reduced sexual activity [4]. Furthermore, oncological outcomes in patients with anastomotic leakage after rectal surgery are far worse, with an increase of local recurrences and a decrease of disease-free survival at 5-year [5]. Despite a more accurate classification, the progress in recognizing and preventing preoperative risk factors and the use of new anastomotic techniques, leakage remains a very topical problem in rectal surgery [3, 6-8]. A crucial point is intraoperative testing of the integrity of the anastomosis. The most frequently used test is the classic air leak test (ALT), which consists of filling the pelvis with a saline solution and manually occluding the proximal bowel. Air is then insufflated through the anus, and if air bubbles are noted in the pelvis, an incomplete anastomosis is suspected. The use of this type of test is a very controversial topic in particular after low rectal dissections in which a low colorectal anastomosis is fashioned or in case of coloanal anastomosis [9]. We developed an original technique used since 2011 [10-12]. We suggested to perform the hydropneumatic test in reverse in the case of a laparoscopic approach to rectal cancer (saline solution in the rectum and CO2 in the abdomen) and to visualize the low anastomoses transanally, with the assistance of devices used for proctological surgery.
The feasibility and safety of our original test have been recently suggested in 2 recent publications [13, 14]. In fact, the authors described the application of the “reverse ALT” in laparoscopy in the field of transanal total mesorectal excision (TME) and coloanal anastomosis.
The aim of this study is to report our experience in the last 5 years with the reverse ALT in anterior rectal resections performed in laparoscopy for low rectal cancer type I according to the Roullier classification [15] and in which a low colorectal anastomosis was fashioned.
METHODS
Study design and study population
This cohort study relies on data retrieved from a prospectively maintained database of consecutive patients undergoing elective rectal resection for adenocarcinoma at the St. Giuseppe Moscati Hospital of Avellino, Italy from October 2014 to November 2019. The database was implemented in 2014 and included preoperative, operative, and postoperative data. All patients signed written informed consent including the possibility of future publication according to the Italian bioethics laws. This study was approved by the Institutional Review Board of the S.G. MOSCATI Hospital (00101062020) in compliance with the principles of the Helsinki Declaration.
Inclusion and exclusions criteria
Only patients who underwent a low rectal resection with a laparoscopic approach for low primary rectal cancer (type I in Roullier classification) [15] were recruited for the study. Patients younger than 18 years of age, pregnant, with recurrent disease, with cancer less than 4 cm from the anal verge, undergoing abdominoperineal resection or emergency surgery were excluded from the study.
Preoperative assessment and preparation
All patients underwent standard preoperative staging for rectal cancer, including colonoscopy with biopsy, computed tomography (CT) chest, CT abdomen and pelvis, magnetic resonance imaging pelvis, and/or endorectal ultrasound. Our colorectal multidisciplinary meetings validated the operative indication.
The day before the surgery, patients received full mechanical bowel preparation with polyethylene glycol and a slag-free diet started 1 week earlier. Thromboembolic prophylaxis with low-molecular-weight heparin was given the evening before the surgery. Antibiotic prophylaxis with second-generation cephalosporin was administered at induction of anesthesia.
Surgical technique
Laparoscopic low anterior resection with TME was performed. Low colorectal anastomosis was performed as described in the TICRANT study [8]. At the beginning of the intervention, a 33-mm circular anal dilator (CAD) device was inserted into the anal canal for 3 cm and fixed to the perianal skin at the 4 cardinal’s points. The rectal inspection was carried out by a 33-mm purse suture anoscope (PSA) of 10 cm in length to correctly identify the proximal and distal margin of the tumor. The rectum section by stapler was performed under direct inspection through the CAD.
Four 2-0 polypropylene sutures were placed; 2 of them at the extremities of the suture line (left and right), and then the stump is pulled through the anus to allow the placement of the other 2 sutures transanally on the rectal stump 1 cm medially to the previous 2 sutures.
The circular stapler was introduced through the CAD (29- or 33-mm KOL stapler, Touchstone International Medical Science Corp., Suzhou, China). The 4 tails of the polypropylene stitches were introduced through the stapler channels (2 on the left and 2 on the right sides of the instrument) and gentle traction was applied in order to obtain a gradual and homogeneous tension of the tissue to eliminate the previous staple line and “dog ears”, to prevent tissue squeezing and crashing.
After that, the anvil was introduced into the proximal colon and the “cul de sac” closed with linear stapler, to perform a side-to-end colorectal anastomosis. Subsequently, the circular stapler was opened, the spike was connected with the anvil, and the stapler was closed. After obtaining good healthy tissue plane the circular stapler was fired, and the competence of “donuts” was examined.
Once the anastomosis was performed, the presence of the 33-mm CAD allowed its adequate anastomotic inspection in all 360°. The 33-mm PSA was inserted through the CAD, allowing the inspection of the low colorectal anastomosis dividing the same in the 30% quadrant.
Subsequently, a saline solution was injected into the rectum. The anastomosis was thus inspected and at the site where the bubbles appeared, an additional interrupted suture in polyglactin 3-0 was used to reinforce the anastomotic line.
Anastomosis was finally evaluated with indocyanine green (ICG) fluorescence angiography. ICG was used in the range of 0.1 to 0.3 mg/kg. After completion of the anastomosis, a bolus of ICG was injected intravenously. A reverse ALT was performed. Subsequently, the entire anastomotic line was examined under near-infrared (NIR) illumination by inserting a second laparoscopic camera (KARL STORZ SE & Co. KG, Tuttlingen, Germany) inside the CAD. Perfusion of both proximal and distal anastomotic mucosal appearance was also assessed and classified according to 3-tier trans-CAD ICG mucosal grading system (MGS) (grade 0, apparently normal perianastomotic mucosa; grade 1, ≤ 30% vascular deficiency or congestion; and grade 2, > 30% vascular deficiency or congestion) (Table 1) inspired to the endoscopic MGS classification proposed by Sujatha-Bhaskar et al. [16].
A drain was left in the pelvis at the end of the procedure and the need for protective stoma was left to the discretion of operating surgeons.
Postoperative course
The patients were fed from the 1st postoperative day (POD) and deperfused if oral analgesic drugs alleviated the pain. The control of the leukocyte formula, hemoglobin, renal function, ionogram, and C-reactive protein (CRP) was performed systematically on POD 1, 3, and 5.
The gastric probe was removed immediately at the end of the surgery and the urinary catheter was removed on POD 3. Drainage was removed from POD 3 if the CRP level was less than 170 mg/L and if the drainage was less than 50 mL of serum-hematic liquid. All patients received 4 weeks’ prophylactic dose of low-molecular-weight heparin. The discharge of patients was authorized starting from POD 6. Complications were classified according to the Clavien-Dindo classification of surgical complications [17].
Definition of anastomotic leak
Anastomotic leak was defined according to definition of International Study Group of Rectal Cancer: “Defect of the intestinal wall integrity at the colorectal or coloanal anastomotic site (including suture and staple lines of neorectal reservoirs) leading to a communication between the intra- and extraluminal compartments. A pelvic abscess close to the anastomosis is also considered as an anastomotic leakage” [7].
The identification of postoperative anastomotic leak was clinical and biologic; fever, tachycardia, signs of local or generalized peritonitis, pus or stools from the drainage tube, and a CRP greater than 170 mg/L on POD 3 was strongly suspected of fistula. In the presence of one or more of these signs, a CT scan was performed to confirm the diagnosis.
The fistulas were classified in grade A (anastomotic leakage requiring no active therapeutic intervention), B (anastomotic leakage requiring active therapeutic intervention but manageable without relaparotomy), and C (anastomotic leakage requiring relaparotomy) [7].
Stoma closure
The stoma closure was scheduled 6 to 8 weeks after surgery if adjuvant chemotherapy was not needed. Otherwise, they were scheduled 3 to 6 months after the surgery. In any case, the preoperative evaluation included a clinical examination, laboratory tests, and an abdominal CT scan with rectal opacification in search of an anastomotic fistula.
Patient’s follow-up and outcome
The patients were controlled 15 days, 1 month after the surgery, and then followed by the oncologist if adjuvant chemotherapy had been necessary. Subsequently, the follow-up consisted of surveillance with tumor markers and clinical examination every 3 months for the first 2 years, subsequently every 6 months for the following 3 years. A total body CT scan was performed annually for the first 5 years. Colonoscopy was performed 9 months after surgery if a complete colonoscopy had not been performed in the preoperative period; otherwise, it was scheduled within the 3rd and 5th year of the surgery.
The primary outcome was the incidence of the anastomotic leak during 30 PODs. Secondary outcomes were overall 90-day postoperative morbidities and mortality, and the stoma closure rate on schedule.
Variables studied and statistical analysis
Patients were identified in a prospectively maintained database and analyzed retrospectively. Basic patients’ demographic data were recorded including age, sex, body mass index (BMI), American Society for Anesthesiologists (ASA) physical status (PS), preexisting pathologies, preoperative nutritional evaluation, use of neoadjuvant chemoradiotherapy, the length of hospital stay, postoperative morbidity, anastomotic leak rate, mortality, and the stoma closure rate. Data were analyzed using Microsoft Excel (Microsoft, Redmond, WA, USA) and IBM SPSS Statistics ver. 24 for Mac (IBM Corp., Armonk, NY, USA). Quantitative data were expressed as median and range.
RESULTS
The characteristics of all patients are shown in Table 2. Eleven patients with low rectal cancer (type I Roullier classification) were included in our study. The average age was 67 years (range, 58 to 78 years), 54.5% were female, and with a BMI of 25 kg/m2 (range, 20 to 35 kg/m2). Seven patients (63.6%) were defined as ASA PS stage II, and 90.9% of patients had preoperative albumin levels within the limits. More than half of the patients (54.5%) underwent neoadjuvant chemoradiotherapy. Laparoscopic TME was performed in all patients as described without any intraoperative complications.
The reverse ALT was performed in 11 patients; in 4 of these (36.4%), the test was positive and the addition of interrupted sutures was necessary (Fig. 1). No really physical defects or disruption were found; rather, leaks through staples were identified. The confirmation test was negative in all cases after repaired.
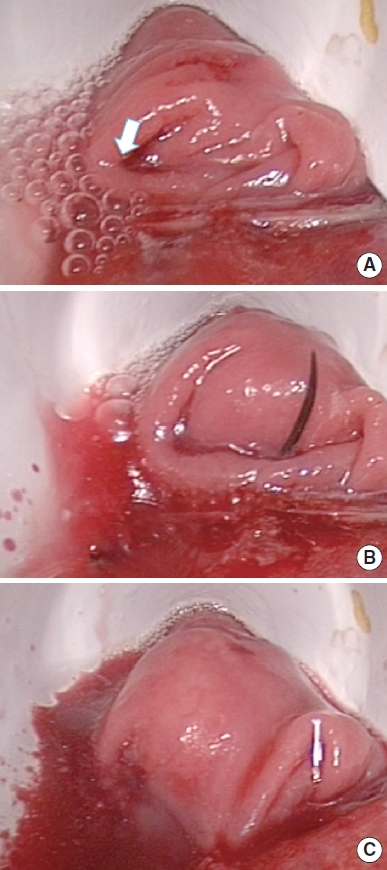
(A) Positive reverse air leak test; the white arrow indicates the bubbles with the defect of the anastomotic line. (B) The defect is repaired with an interrupted vicryl 3.0 suture. (C) The confirmation test shows the repair of the defect with the disappearance of the bubbles.
In all patients after performing the anastomosis, the anesthesiologist injected a bolus of ICG. Colorectal anastomosis was evaluated under NIR illumination and scored according to our classification (grade 0 to 2). In all patients, a good perfusion of the tissues and a good vitality of the mucosa were found (grade 0) (Fig. 2). There were no side effects or allergic reactions related to the injection of ICG. A protective stoma was performed in 10 patients (90.9%).
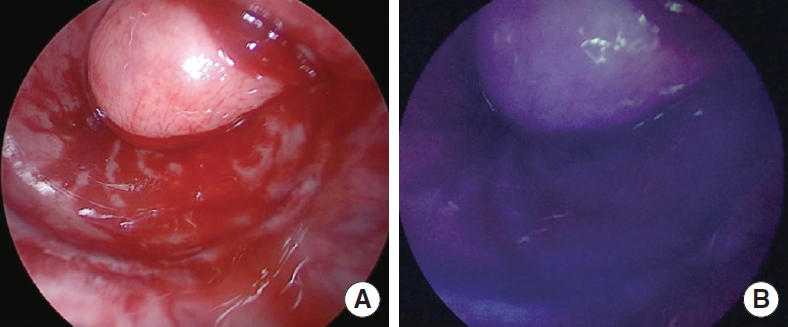
(A) Inspection of the anastomosis integrity and the proximal and distal mucosa. (B) Inspection of the integrity of the anastomosis with near-infrared illumination after the intravenous injection of indocyanine green.
The postoperative course was marked by 2 minor complications; a postoperative ileus, which resolved spontaneously after 48 hours, and an acute urinary retention. There was no anastomotic fistula in the patient cohort. The median length of hospital stay was 7 days (6 to 10 days). There was no readmission or death in the 90 days following the surgery. Stoma closure was performed on schedule for all patients.
DISCUSSION
To the best of our knowledge, we were the first ones to describe this approach [10-12] and the first to describe more than 10 consecutive patients tested with the reverse ALT in laparoscopic rectal resection for low rectal cancer (type I Roullier). Other authors subsequently confirmed the validity of this technique in transanal TME procedure and coloanal anastomosis [13, 14].
Yassin et al. [14] reported the case of a patient who underwent transanal TME for low rectal cancer. The integrity of the anastomosis was assessed with the reverse ALT, which allowed to identify a defect in the posterior site; therefore, it was repaired and a second test confirmed the absence of leaks. A temporary ileostomy was performed and no postoperative complications were reported.
Emile and Wexner [13] described a different type of reverse ALT in order to verify the integrity of the coloanal anastomosis; after the anastomosis was performed, the pelvis was filled with saline solution and the patient was placed in the reverse Trendelenburg position. During laparoscopy, pneumoperitoneum pressure forced fluid mixed with gas between the sutures. The escape of the fluid made it possible to identify the defect in the anastomosis line, which could therefore be reinforced.
The validity of the intraoperative ALT is a hotly debated topic in the literature. A recent systematic review and meta-analysis [9], which included 20 articles of which 2 randomized trials [18, 19] and 5,283 patients, showed a lower rate of anastomotic leak in the group of patients who underwent air leak testing compared to those not subjected, although this value was not statistically significant. However, patients with a positive test possessed a significantly higher anastomotic leakage rate than the patients with negative test. So, in other words, the ALT would not be able to reduce the risk of developing an anastomotic leak but would allow recognizing patients at high risk of developing a postoperative leak [9]. However, this systematic review and meta-analysis had several limitations: selection bias (in some series the ALT was performed at the discretion of the surgeon), heterogeneous methodology to test the colorectal anastomosis, and bias in the interpretation of the current evidence. The only 2 randomized studies and a recent retrospective study of over 700 patients demonstrated the ability of the intraoperative ALT to identify the anastomotic leak that could therefore be effectively managed intraoperatively, leading to a significantly lower risk of the postoperative leak [18-20].
The intraoperative positive rate of classic ALT varies from 1.5% to 24.7% among different studies and approximately, 4% of patients developed an anastomotic leak despite test negativity [9]. Reverse ALT had a higher positivity rate (> 30%); therefore, it would appear to have a greater sensitivity. However, larger studies are imperative to validate this assertion.
Although the classic ALT has been used for many years, this procedure has never been standardized. Many surgeons inject 60 mL of air and another 400 mL; but in fact, no study is available to verify the volume of air needed to test the anastomosis or the amount of saline solution to be introduced into the pelvis [9]. Much depends on the anatomical conditions and, anyway, classic ALT remains an operator-dependent test. The reverse ALT, on the contrary, can be easily standardized; few mL of saline solution and a constant pneumoperitoneum are sufficient to perform the test.
As resumed by Nachiappan et al. [21], there are several types of procedures able to evaluate intraoperatively the integrity of colorectal anastomosis. They can be divided into 3 categories; (1) basic mechanical patency assessment techniques, (2) endoscopic visualization techniques, and (3) microperfusion techniques. In the first category, which included the classic ALT, saline leak and methylene blue leak tests, randomized and nonrandomized controlled study showed that the risk of a postoperative anastomotic leak was significantly less in the intraoperatively tested group compared to the non-tested group. As concerning the second group, i.e. performing an intraoperative coloscopy, no randomized trials were available. Including only nonrandomized studies, this meta-analysis showed no statistically significant difference between postoperative complication rates in the intraoperative colonoscopy group and control groups (P = 0.30). Furthermore, the postoperative complication rate was similar in the positive test and in the negative test group. However, the number of postoperative complications in the positive group may have been lowered by the intraoperative measures undertaken. Yang et al. [22], in a propensity score matching study, showed that the rate of anastomotic fistulas was higher in the group in which intraoperative coloscopy had not been performed (4.3% vs. 11.7%, P = 0.007). Anyway, the execution of an intraoperative coloscopy is often difficult to organize, it requires specific skills that not all surgeons have. Finally, the category of microperfusion techniques included 9 different procedures and among these, the NIR fluorescence angiography with ICG. The principle is to identify the vitality of the tissues, identifying areas of hypoperfusion.
Rausa et al. [23] in a recent systematic review and network meta‐ analysis, including 11 articles, showed that the anastomotic leak rate was significantly higher in the control group (no test) than in the ICG group and higher, but not statistically significant, in the classic ALT and intraoperative colonoscopy groups.
However, to date, no randomized study on this topic has shown a real advantage in terms of postoperative morbidity and mortality. Furthermore, the cost of equipment to perform fluorescence angiography remains a limit for many institutions.
In our experience, ICG-NIR was used in all patients tested with the reverse ALT; and in all cases, a good tissue perfusion was found. Taking a cue from Sujatha-Bhaskar et al. [16], we applied the endoscopic classification to the trans-CAD MGS diving patients in 3 groups: (1) grade 0, apparently normal perianastomotic mucosa; (2) grade 1, ischemia or congestion involving ≤ 30% of either the colon or rectal mucosa; (3) grade 2, ischemia or congestion involving > 30% of the colon or rectal mucosa or ischemia/congestion involving both sides of the staple line. Therefore, in our series, the ICG-NIR showed normal tissue perfusion even in patients with positive reverse ALT test. This result leads us to think that the 2 methods can be used in a complementary way in order to identify the weaknesses in the anastomosis.
In the literature, there is no real single agreement on the management of a positive ALT; oversewing, additional interrupted sutures, temporary stoma, defunctioning stoma, or reanastomoses [9, 18, 19, 24]. By combining the result of the reverse ALT with the angiographic classification proposed, we developed a decision algorithm for each case (Fig. 3). In this way, in our 4 patients with positive reverse ALT, an interrupted suture and protective stoma were performed and no postoperative leaks occurred.
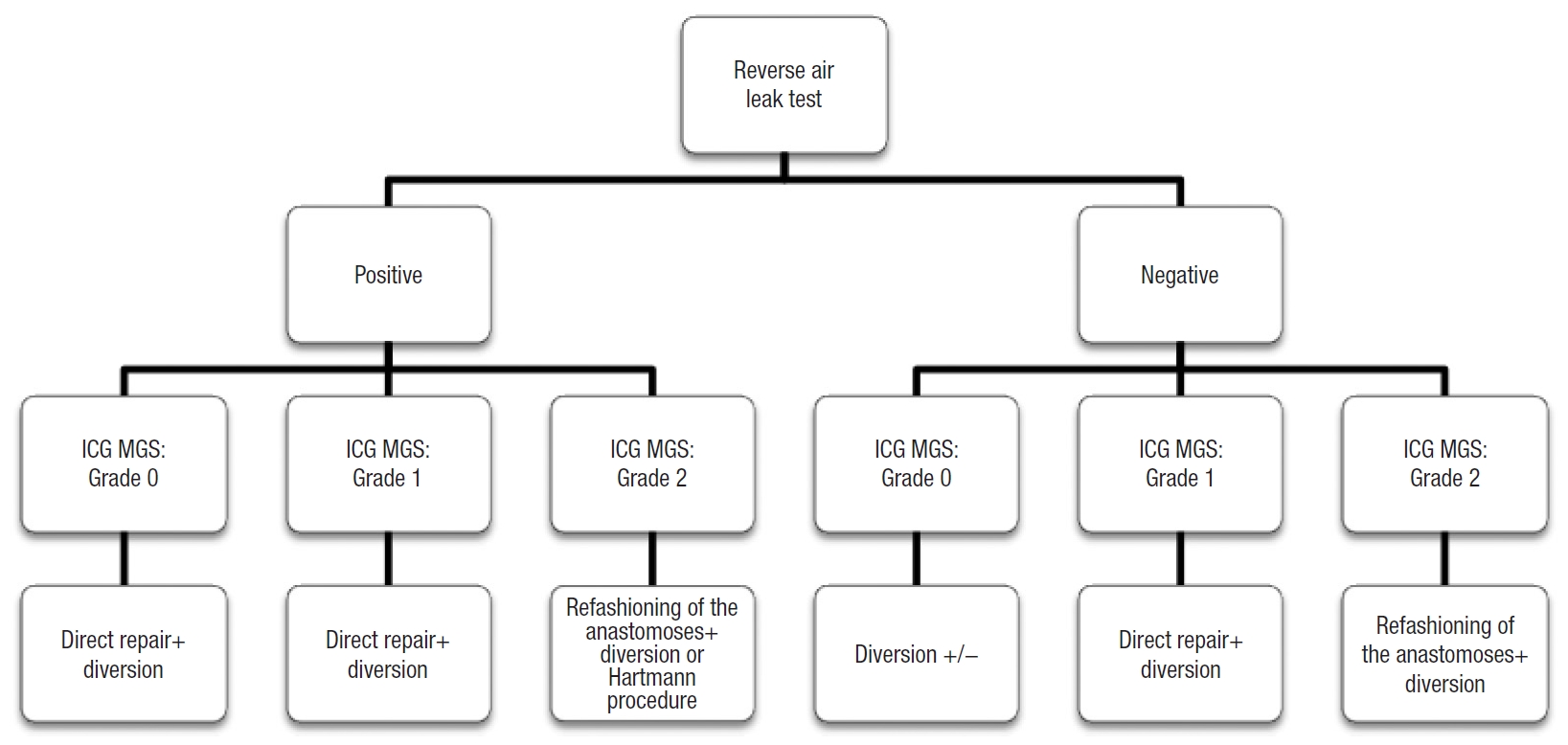
Decision algorithm that combines the result of the reverse air leak test with the indocyanine green (ICG) mucosal grading system (MGS).
Five thinks have to be underlined: (1) the PSA allowed us to divide the low colorectal anastomosis precisely into the portion of 30% each; (2) the PSA is transparent so while exploring 30% of the low colorectal anastomosis we can perfectly assess the perfusion of the remaining 70% of the suture line; (3) we found a 36.3% of patients with a positive reverse ALT, all of them had a grade 0 trans-CAD ICG MGS and subsequently, due to the absence of perfusion defects, patients underwent a direct trans-CAD repair with sutures; (4) no postoperative fistula was detected in our study in comparison with the results reported by Sujatha-Bhaskar et al. [16] (about 9%); (5) unlike the classic ALT, the transanal approach allows an adequate visually inspection of the staple line, allowing to identify bleeding sites and mucosal ischemia or disruption. Nevertheless, we have to consider that inevitably if the perfusion defect is superior (grade 1 or 2 in ICG MGS), the fistula rate could increase and the management of the anastomosis could be different according to algorithm proposed (Fig. 3). The result of the reverse ALT did not change our conduct on the stoma confection, because it was not the main aim of our article and for ethical reasons. In any case, the absence of bubbling, a good anastomotic perfusion (grade 0) and the absence of disruption could lead to the choice of the absence of stoma confection. However, this assumption has to be validated with specific studies.
This is the only study available in the literature on this new technique that includes a group of patients. On the other hand, our study has several limitations; first of all, the number of participants was limited to only 11 patients and this was the experience of a single surgeon in a single center. Moreover, this was a retrospective observational study and no comparison with a control group was performed.
In conclusion, the reverse ALT is a technique for the assessment of low colorectal anastomosis, easy to perform, reproducible, and safe. Its ability to detect leaks would seem satisfactory but further studies should be conducted to evaluate its real effectiveness. In addition, the evaluation of colorectal anastomosis with fluoroangiography could be easily associated in order to recognize a defect in the anastomotic line that can be scored according to this new trans-CAD mucosal perfusion grading system. Other case-control studies that include a larger sample size are indispensable to validate the efficacy of this technique.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.


