Intraoperative fluorescence angiography as an independent factor of anastomotic leakage and a nomogram for predicting leak for colorectal anastomoses
Article information
Abstract
Purpose
Colorectal anastomotic leakage (AL) is a life-threatening complication, which increases morbidity, hospital stay and cost of treatment. The aim of this study is to identify risk factors, including intraoperative indocyanine green fluorescence angiography (ICG FA), associated with the leak of stapled colorectal anastomosis.
Methods
Four hundred twenty-nine consecutive patients underwent surgery between 2017 and 2019 for benign (n=10, 2.3%) or malignant (n=419, 97.7%) and rectal (n=349, 81.4%) or distal sigmoid (n=80, 18.6%) lesions with double-stapling technique reconstruction were included into retrospective study. Univariate analysis and multivariate logistic regression of the tumor-, patient- and treatment-related risk factors of AL was performed.
Results
An AL developed in 52 patients (12.1%). In multivariate analysis following variables were independently associated with AL; male sex (odds ratio [OR], 3.8; 95% confidence interval [CI], 1.9−7.7; P<0.01), anastomosis at ≤6.5 cm from anal verge (OR, 3.1; 95% CI, 1.3−7.5; P=0.01), and age of ≤62.5 years (OR, 2.1; 95% CI, 1.1−4.1; P=0.03). ICG FA was found as independent factor reducing colorectal AL rate (OR, 0.4; 95% CI, 0.2−0.8; P=0.02). A nomogram with high discriminative ability (concordance index, 0.81) was created.
Conclusion
ICG FA is a modifiable surgery-related risk factor associated with a decrease of colorectal AL rate. A suggested nomogram, which takes into consideration ICG FA, might be helpful to identify the individual risk of AL.
INTRODUCTION
Colorectal anastomoses became a routine procedure in surgery after introduction of circular staplers (Russian gun) in the late 1960s [1] and the double-stapling technique in the 1980s [2]. Despite the advances in surgical technique and perioperative management of patients, an anastomotic leakage (AL) remains urgent problem of colorectal surgery [3, 4].
The main prerequisites for successful anastomosis are adequate blood supply, good stapling technique, and absence of tension between anastomosed intestinal segments. There are a number of AL risk factors [5-7] mentioned in a number of published papers. Though a lot of them are conflicting, such risk factors as level of anastomosis over anal sphincter, sex, obesity, smoking, and use of steroids, preoperative chemoradiotherapy (CRT) as well as intraoperative blood loss and postoperative blood transfusion are considered as significant in most of the published series. On the other hand, reinforcement of anastomosis [8], transanal tube [9, 10], or biodegradable soft sheath (C-seal) [11] of the anastomosis remains the methods with unproven effectiveness. In recent years, the new method, indocyanine green (ICG) fluorescence angiography (FA), was introduced into surgical practice for blood supply quality assessment. The Pillar II Study was demonstrated an AL rate of 1.9% for low anastomoses and/or after pelvic radiation [12]. Another phase II multicenter study [13] reported on AL rate of 3% for low colorectal anastomoses. This is a very low rate of AL.
Though AL is hardly ever will be avoided entirely, a good prediction tool would be helpful for surgeon and patient decision to avoid anastomosis at all. Several nomograms have been developed to predict AL after surgical treatment of rectal cancer [14-16]. The purpose of presented retrospective single-center study was to develop a new nomogram for prediction of AL of colorectal anastomosis, which included novel method of ICG FA.
METHODS
A retrospective analysis of database collected prospectively between March 2017 and August 2019 was performed. The inclusion criteria was the formation of a colorectal anastomosis with a double-stapling technique for benign or malignant tumors of rectum or distal sigmoid. All operations were conducted by 3 colorectal surgeons who perform more than 50 colorectal resections per year. Ethical approval for the trial protocol was obtained from the local independent ethics committee of State Scientific Centre of Coloproctology (No. N80, 02.02.2017). All participants provided written informed consent prior to enrolment in the study.
An anastomotic leak was defined in accordance with proposal of the International Study Group of Rectal Cancer [17]. An AL was defined as any defect of the colorectal anastomotic site leading to a communication between the intra- and extraluminal compartments. It was graded as follows: grade A is an AL requiring no active therapeutic intervention, grade B is AL requiring active therapeutic intervention but manageable without relaparotomy, and grade C is AL requiring relaparotomy. The clinical and radiological AL was confirmed by contrast enema or computed tomography scan in all patients within 30 days after surgery.
Since March 2017, we started to use ICG FA for assessment of the perfusion of the anastomosis. A sterile water-soluble lyophilized powder of ICG (Pulsion Medical Systems, Munich, Germany) was used. After the surgeon’s selection of the transection level of the colon for anastomosis of surgeons ICG was injected intravenously at a dosage of 0.2 mg/kg. A FA was performed using laparoscopic system (Karl Storz SE & Co., KG, Tuttlingen, Germany) 2 to 3 minutes after ICG injection. If uniform blue glow emission was detected from ICG injection, the site of colon chosen for anastomosis creation would estimate as well perfused. If the fluorescence was equivocal or absent the colon was regarded as poorly perfused and surgeon had to change a transection line in a proximal direction up to the level of adequate blood supply. For reinforcement anastomosis, we placed 6 to 8 interrupted sutures along the staple line circumferentially.
Statistics
The univariate associations were tested with Pearson chi-square test. A P-value less than 0.05 was considered statistically significant. All independent variables with P≤0.05 in the univariate analysis were subsequently included in a multivariate stepwise logistic regression model to determine risk factors associated with AL after controlling for covariates with P<0.1. Optimal cutoff points were defined by the receiver-operating curve analysis with Youden index calculation. Statistical analysis was performed using GraphPad Prism ver. 8.3 (GraphPad Software, La Jolla, CA, USA). The nomogram of AL was created based on multivariate logistic regression analysis and constructed using the “rms” package (http://www.r-project.org/).
RESULTS
Results of 429 resections for rectal lesion with colorectal anastomosis were analyzed. Table 1 shows patients’ demographic, clinical, and surgery characteristics. Of 429 tumors, 349 (81.4%) were located in rectum and 80 (18.6%) localized above 15 cm from anal verge as measured by rigid proctoscope. Forty-six of rectal carcinomas (10.7%) underwent neoadjuvant CRT of 45 to 54 cGy in 4 to 5 weeks with 5-fluorouracil or capecitabin. Subsequent surgery was performed 6 to 10 weeks later. In 40 cases (9.5%), stage IV of colorectal cancer was diagnosed due to synchronous liver metastases. Simultaneous metastasectomy was performed in 20 of them and the rest of the patients with hepatic lesions were scheduled for subsequent liver surgery. Resection of adjacent organs was necessary in 49 patients (11.4%) and a final pathology demonstrated R0 resection in 403 (93.9%).
ICG FA was performed in 239 patients (55.7%) and resulted in the change of transection line in 50 of 239 cases (20.9%).
All patients had stapled colorectal anastomosis and median distance of anastomosis from anal verge was 8 cm (range, 4–15 cm). Of them, 252 of 429 (58.7%) located between 4 and 8 cm and 177 of 429 (41.3%) located between 9 and 15 cm. Air leak test of anastomosis performed in 360 of 429 patients (83.9%) and it was positive in 54 of 360 patients (15.0%). Reinforcement of anastomosis was done in 223 of 429 (52.0%) patients and defunctioning ileostomy was fashioned during surgery in 307 patients (71.6%).
An AL was developed in 52 patients (12.1%). The rate of AL grade A (asymptomatic radiological) was 7.5% (32 of 429). The rates of AL grade B and C were 3.0% (13 of 429) and 1.6% (7 of 429), respectively. The median postoperative hospital stay was 9 days (range, 4–32 days).
In the univariate analysis (Fig. 1) of patient-related risk factors of AL, male sex (odds ratio [OR], 4.1; 95% confidence interval [CI], 2.1−7.9; P=0.001) and age of ≤ 62.5 years (OR, 1.9; 95% CI, 1.0−3.4; P=0.04) were associated with a higher risk of AL, while body mass index, American Society of Anesthesiologists physical status classification, diabetes mellitus, and smoking had no influence on AL (P>0.05). Among treatment-related risk factors preoperative CRT (OR, 3.0; 95% CI, 1.4−6.3; P=0.003), total mesorectal excision (OR, 7.9; 95% CI, 3.1−20.3; P=0.001), colorectal anastomosis located at ≤ 6.5 cm from anal verge (OR, 5.7; 95% CI, 2.9−11.1; P=0.001), R1 resection (OR, 2.9; 95% CI, 1.1−7.3; P=0.02) and the formation of a preventive stoma (OR, 7.5; 95% CI, 2.3−24.7, P=0.001) demonstrated significant association with AL. On the other hand, such factors as reinforcement of anastomosis (OR, 0.4; 95% CI, 0.2−0.8, P=0.01) and FA (OR, 0.5; 95% CI, 0.3−0.9; P=0.02) significantly decrease the rate of AL. In our opinion, the high frequency of AL in patients with a preventive stoma is associated with the need to form a stoma in patients with a high-risk AL (for example, low anastomoses and it is a matter of statistics). Preventive stoma did not have an independent effect on the AL rate, which was confirmed by the results of multivariate analysis.

Flowchart of univariate analysis of factors related to the anastomotic leakage (AL). OR, odds ratio; CI, confidence interval; AV, anal verge; CRT, chemoradiotherapy; ASA, American Society of Anesthesiologists; PS, physical status; BMI, body mass index; FA, fluorescence angiography.
In multivariate analysis (Fig. 2), independent variables associated with increase of AL rate were male sex (OR, 3.8; 95% CI, 1.9−7.7; P<0.001), low anastomosis (OR, 3.1; 95% CI, 1.3−7.5, P=0.01) and age of ≤ 62.5 years (OR, 2.1; 95% CI, 1.1−4.1; P=0.03). Only ICG FA was found as independent factor reducing colorectal AL (OR, 0.4; 95% CI, 0.2−0.8; P=0.01).
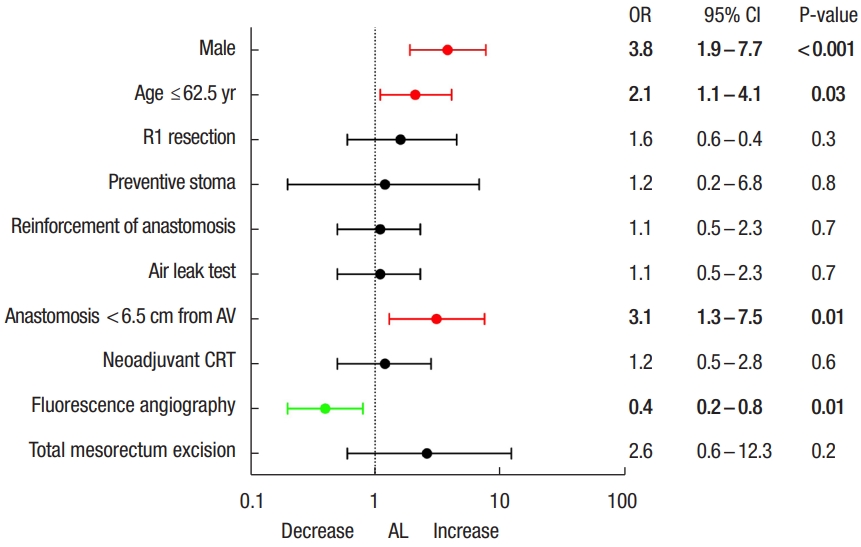
Flowchart of multivariate analysis of factors related to anastomotic leakage (AL). OR, odds ratio; CI, confidence interval; AV, anal verge; CRT, chemoradiotherapy.
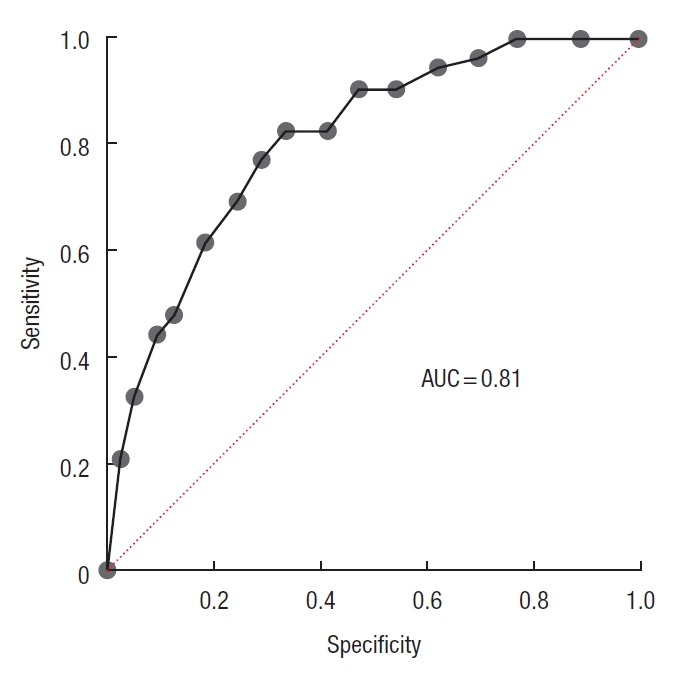
The nomogram of AL (Fig. 3) was created based on multivariate logistic regression analysis. The area under the curve was 0.81 (95% CI, 0.75−0.87; P=0.0001) (Fig. 4) and the concordance index was 0.81, that indicating well predictive ability.
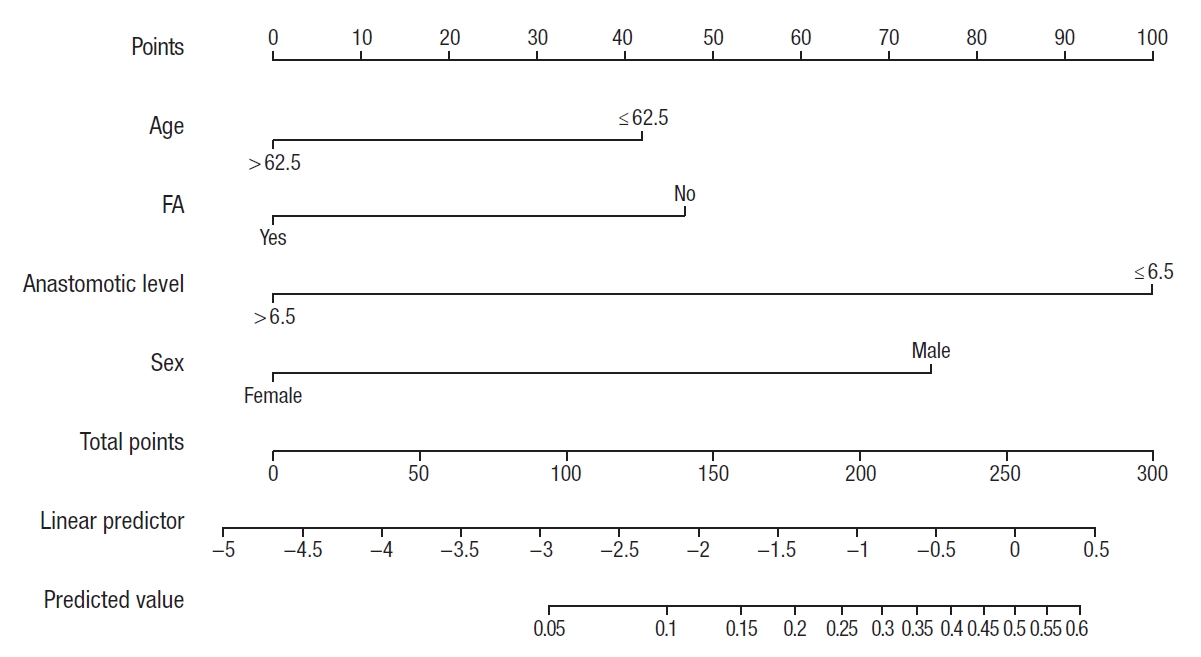
A nomogram for predicting individual risk of leakage for colorectal anastomosis. After projecting a vertical line on the scale ‘points,’ the total sum is calculated. Projection of vertical line from the scale ‘total points’ to ‘predictive value’ gives the probability of anastomotic leakage. FA, fluorescence angiography.
DISCUSSION
The presented retrospective study demonstrated AL rate of all grades for colorectal anastomosis of 12.1%. Seven variables were associated with increased risk of AL and 3 with decrease risk in univariate analysis. However logistic regression allowed to identify only 4 independent risk factors of AL. Two of them, i.e., male sex and age younger than 62 years, were patient-related. According to the meta-analyses of Pommergaard et al. [18], which included 110,272 patients from 23 studies, male sex (OR, 1.48; 95% CI, 1.37−1.60) is likely to be a risk factor for AL, though the quality of the evidence was low. In our study male sex was the most significant independent factor (OR, 3.8; 95% CI, 1.9−7.7; P<0.01), which can be explained by prevalence of rectal carcinomas in presented series of patients, where male narrow pelvis and complex anatomy may contribute to technical difficulties with the formation of colorectal anastomosis [19].
Another patient-related factor associated with increased risk of AL in presented study was the age of ≤ 62.5 years. The abovementioned meta-analyses [18] revealed that age (OR, 0.99; 95% CI, 0.89–1.10) was not a risk factor for AL. Contrary, Zaimi et al. [20] in his study, demonstrated the increase of incidence of colorectal AL with decreasing age: in patients ≥ 80 years old, 4.9%; 70 to 80 years old, 5.4%; 60 to 69 years old, 5.5%; and < 60 years old, 6.4% (P<0.001). Multivariate analysis showed that age was protective for AL (OR, 0.96 per 5 years; 95% CI, 0.94–0.98; P<0.001). In accordance with the results of an experimental study [21], aging tissues demonstrate more chronic inflammation. Hypothetically, chronic inflammation in aging tissues enables anastomotic healing preventing an exceeded inflammatory response [22]. However, considering the presence of vascular disorders in the mesentery of the colon in elderly patients (for example, atherosclerosis), ICG FA is necessary for an adequate assess the perfusion of the anastomosis.
In terms of surgery-related risk factors only low anastomosis, i.e. below 6.5 cm from anal verge, in our study was associated with a higher rate of AL (OR, 3.1; 95% CI, 1.3−7.5; P=0.01). The factor of low anastomosis [3, 18] or low rectal tumor [5-7] is the most univocal in literature.
Surgeon’s assessment of risk of AL demonstrated a low predictive value of 62% of sensitivity and 52% of specificity [23]. Intraoperative air or dye test of anastomotic integrity is one of the most reproducible in routine colorectal surgery [24]. Interestingly, that air leak test per se demonstrated strong tendency to reduce AL (OR, 0.5; 95% CI, 0.3−1.0; P=0.06) in presented study, though if test was positive it played no role for AL (OR, 1.0; 95% CI, 0.4−1.0; P=0.9). One plausible explanation for this finding is that the positive leak test urges surgeon to reinforce the line of stapled anastomosis by additional hand sutures or create defunctioning stoma.
Besides the leak-proof stitches and no-tension between anastomosed bowel ends, good blood perfusion of intestinal wall is a prerequisite of successful healing. Conventional assessment of intestinal perfusion based on evaluation of pulsation of vessels, bowel color, and bleeding from the marginal artery is very subjective.
Up to date, the results of 2 meta-analyses of the use of ICG FA in colorectal surgery available [25, 26]. The meta-analysis of Blanco-Colino and Espin-Basany [25] combining results of 5 non-randomized studies and 1,302 patients showed that FA in colorectal cancer surgery significantly reduced the AL rate (OR, 0.34; 95% CI, 0.16–0.74; P=0.006) and even greater reducing rate of AL in rectal cancer surgery (OR, 0.19; 95% CI, 0.05–0.75; P=0.02). According to Shen et al. [26] significant benefit in reducing the incidence of colorectal AL of FA was also identified (OR, 0.27; 95% CI, 0.13–0.53; P<0.001). Results of our study also confirm that ICG FA is a modifiable independent factor reducing an incidence of colorectal AL (OR, 0.4%; 95% CI, 0.2−0.8; P=0.01).
An obvious limitation of this study is its single-center and retrospective design. On the other hand, the homogeneous population of patients, i.e. all had colorectal anastomoses, which are most vulnerable for leak is a strength of our study. Also, we evaluated AL, including radiologic AL, at 30-day follow-up and this allowed to get an actual rate of AL even in the presence of diverting ileostomy. Up to our knowledge, the suggested nomogram is the first one, which takes into consideration such factor as ICG FA.
In conclusion, this study showed that male sex and anastomosis below 6.5 cm from anal verge were independent risk factors of colorectal AL. Age over 62.5 years and ICG FA had protective effect on colorectal anastomosis. A suggested nomogram, which takes into consideration ICG FA, might be able to identify the risk of AL for colorectal anastomosis.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.