Comparison of long-term outcomes of colonic stenting as a “bridge to surgery” and emergency surgery in patients with left-sided malignant colonic obstruction
Article information
Abstract
Purpose
Long-term oncologic outcomes of colonic stenting as a “bridge to surgery” in patients with left-sided malignant colonic obstruction (LMCO) are unclear. This study was performed to compare long-term outcomes of self-expandable metal stent (SEMS) insertion as a bridge to surgery and emergency surgery in patients with acute LMCO.
Methods
This retrospective cohort study included patients with acute LMCO who underwent SEMS insertion as a bridge to surgery or emergency surgery. The primary outcomes were 5-year disease-free survival (DFS), overall survival (OS), and recurrence rate. Survival outcomes were determined using the Kaplan-Meier method and compared using log-rank tests.
Results
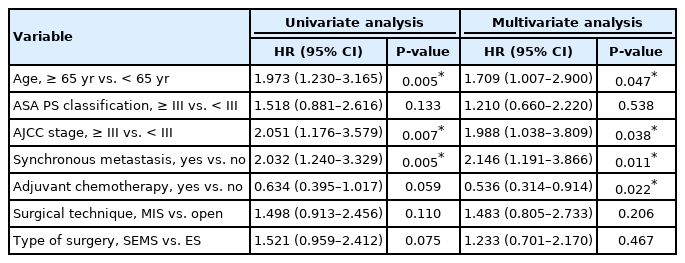
There was a trend of worsening 5-year OS rate in the SEMS group compared with emergency surgery group (45% vs. 57%, P=0.07). In stage-wise subgroup analyses, a trend of deteriorating 5-year OS rate in the SEMS group with stage III (43% vs. 59%, P=0.06) was observed. The 5-year DFS and recurrence rate were not different between groups. The overall median follow-up time was 58 months. On multivariate analysis, age of ≥65 years and American Joint Committee on Cancer stage of ≥III, and synchronous metastasis were significant poor prognostic factors for OS (hazard ratio [HR], 1.709; 95% confidence interval [CI], 1.007–2.900; P=0.05/HR, 1.988; 95% CI, 1.038–3.809; P=0.04/HR, 2.146; 95% CI, 1.191–3.866; P=0.01; respectively).
Conclusion
SEMS as a bridge to surgery may have adverse oncologic outcomes. Patients in the SEMS group had a trend of worsening 5-year OS rate without higher recurrence.
INTRODUCTION
Colorectal cancer (CRC) is the third most common malignant disease worldwide, with more than 1.8 million new cases and 881,000 deaths in 2018 [1]. It has been estimated that 7% to 29% of patients with CRC present with colonic obstruction [2]. The clinical outcomes after resection of patients who present with colonic obstruction are worse than those of patients who present without obstruction. The mortality rate was higher in patients with obstructed CRC than in those without obstruction (17% vs. 6%, respectively) [3].
There is ongoing debate on the optimal approach to the treatment of patients with left-sided malignant colonic obstruction (LMCO). The mortality and morbidity rates for emergency surgery are 15% to 20% and 45% to 50%, respectively, as opposed to a mortality rate of 0.9% to 6% for elective surgery [4, 5]. The reasons for the high morbidity and mortality of emergency surgery are advanced stage of neoplasm, electrolyte imbalances, malnutrition, friable mucosa due to distention, and fecal loading of the unprepared colon [6].
The concept of self-expandable metal stent (SEMS) insertion as a “bridge to surgery,” which converts an emergency situation to an elective one, is appealing. Besides colonic decompression, SEMS insertion allows for preoperative bowel preparation and makes elective single-stage colonic resection possible with decreased risk of permanent stoma creation. In previous randomized controlled trials, SEMS insertion showed favorable short-term outcomes with lower morbidity and permanent stoma rates [7-11].
However, the long-term oncologic outcomes in patients with curable diseases are unclear. Shear forces induced by the SEMS could lead to dissemination of cancer cells into the peritoneal cavity, lymphatic fluid, and bloodstream [12, 13]. A few studies reported poor oncologic outcomes in patients who underwent SEMS insertion, especially in those with SEMS-related perforation [14, 15]. A Japanese nationwide study also reported significantly poorer overall survival (OS) rates in patients who underwent SEMS as a bridge to surgery than emergency surgery [16]. In contrast, many studies reported high success rates and comparable oncologic outcomes for SEMS insertion [17-23].
Because of these inconsistent findings, additional research is needed on SEMS insertion as a bridge to surgery in patients with LMCO. This study aimed to compare long-term oncologic and perioperative outcomes of SEMS insertion as a bridge to surgery and emergency surgery in patients with curable LMCO.
METHODS
Study design and population
We conducted a single-center retrospective study using a prospectively maintained endoscopy database of patients with acute LMCO. We included all patients who underwent surgical resection with curative intent on an intention-to-treat basis at King Chulalongkorn Memorial Hospital between January 2008 and December 2014. LMCO was defined as the presence of at least 1 obstructive symptom (distended abdomen, obstipation, nausea/vomiting) and radiological (dilated colon proximal to the tumor) or endoscopic findings of malignant colonic obstruction between the splenic flexure and rectosigmoid junction. The tumor location of the enrolled patients was defined with abdominal computed tomographic scan by the radiologists. Patients who underwent an intervention with palliative intent or had signs of peritonitis or perforation, colonic ischemia, previous colonic stenting, or contraindication to endoscopic treatment were excluded. The on-call consultant colorectal surgeon decided whether to perform SEMS insertion as a bridge to surgery or surgical intervention after discussion with the patients. The main factors in decision making were the patient’s financial status and medical reimbursement. In Thailand, SEMS insertion has been covered for reimbursement only in patients with the Civil Servant Medical Benefit Scheme. Patients with other medical benefit schemes must pay 1,000 US dollars for a SEMS if they undergo colonic stenting.
The study protocol was approved by the Institutional Review Board of Chulalongkorn University (No. 197-63). Due to the retrospective design of the study, the requirement for consent was waived by the ethics committee.
SEMS insertion technique
All procedures were performed in the operation theatre under conscious sedation. We inserted the SEMS under fluoroscopy-guided direct endoscopic visualization. We did not dilate the stricture site before SEMS insertion. Following colonoscopic assessment of the obstructed site, a 0.89-mm soft-tipped hydrophilic Jagwire (Boston Scientific) was passed through the strictured lumen under fluoroscopic guidance. We did not use enteral contrast to calculate the length of the SEMS. At our center, we only use uncovered colonic stents of 1 size, i.e., 120 mm in length and 24 mm in diameter (Niti-S D-type, Taewoong Medical Corp). We assessed the obstructed lesion preoperatively using computed tomography to ensure that a SEMS that was 120 mm in length would be adequate. During deployment, we focused on the distal end of the stent that was placed 30 mm distal to the tumor and monitored the shape of the proximal end of the stent using fluoroscopy. The distance from the tumor to the distal end of the stent decreased to 20 mm after complete deployment due to foreshortening of the SEMS. After successful SEMS insertion, we attempted to perform colonic resection within 2 weeks as recommended. The surgery performed after SEMS insertion was determined by the consultant colorectal surgeons.
Surgical intervention for left-sided malignant colonic obstruction
Patients undergoing emergency surgery were operated on as soon as possible after initial stabilization. Surgical options included laparoscopic or open resection, subtotal/total colectomy, and segmental resection with or without on-table colonic lavage. Primary anastomosis and stoma formation were at the discretion of the consultant colorectal surgeons.
Outcomes and definitions
The primary outcomes of this study were long-term oncologic outcomes, including disease-free survival (DFS) and OS, and recurrence rate in the SEMS and emergency surgery groups.
DFS was defined as the interval from the date of surgery to cancer recurrence, death, or the last follow-up. OS was defined as the interval from the date of surgery to death or the last follow-up. Recurrence was defined as the development of any new malignant lesion within (locoregional recurrence) or outside (distant recurrence) the field of surgery after curative-intent resection.
The secondary outcomes were perioperative outcomes, including morbidity and mortality, technical and clinical success rates of SEMS insertion, temporary and permanent stoma rates, primary anastomosis rate, adjuvant chemotherapy access rate, and length of hospital stay. Postoperative complications were categorized using the Clavien-Dindo classification [24].
Technical success was defined as successful stent deployment with fluoroscopic confirmation. Clinical success was defined as resolution of obstructive symptoms with stool/flatus passage and oral diet tolerance.
Statistical analysis
Data were analyzed using Stata ver. 15.1 (Stata Corp). The distribution of the data was determined using the De Agostino-Pearson omnibus normality test. Normally distributed data were presented as means and standard deviations, and nonparametric data were presented as medians and interquartile ranges (IQR). Continuous variables were compared using 2-tailed Student t-tests, and categorical variables were compared using the 2-tailed chi-square tests or Fisher exact test, as appropriate. Survival outcomes were determined using the Kaplan-Meier method and compared using log-rank tests. Prognostic factors for OS were assessed using univariate and multivariate Cox proportional hazard models. All analyses were conducted based on the intention-to-treat principle. A P-value of < 0.05 was considered to be statistically significant.
RESULTS
Between January 2008 and December 2014, 126 patients who underwent curative-intent surgery for LMCO fulfilled the eligibility criteria. Of them, 49 underwent SEMS insertion as a bridge to surgery, and 77 underwent emergency surgery (Fig. 1).

Flow chart of enrollment of patients with left-sided malignant obstruction who underwent surgery with curative intent. SEMS, self-expandable metal stent.
Patient characteristics
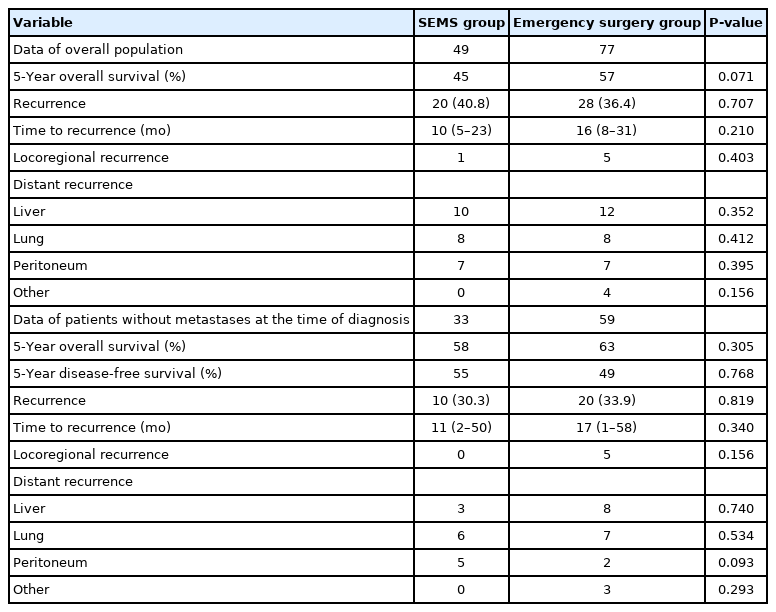
Table 1 shows the baseline characteristics of all 126 patients. The patients in the SEMS group were older than those in the emergency surgery group (mean age, 68 years vs. 61 years; P=0.01). There were no significant between-group differences in sex, American Society of Anesthesiologists physical status classification, tumor stage, tumor location, follow-up time, and adjuvant chemotherapy access rate. However, patients in the SEMS group were more likely to receive the oxaliplatin-based chemotherapy with or without antiangiogenic drug (P=0.03). The median follow-up time for all patients was 58 months (IQR, 23–94 months).
Primary outcomes
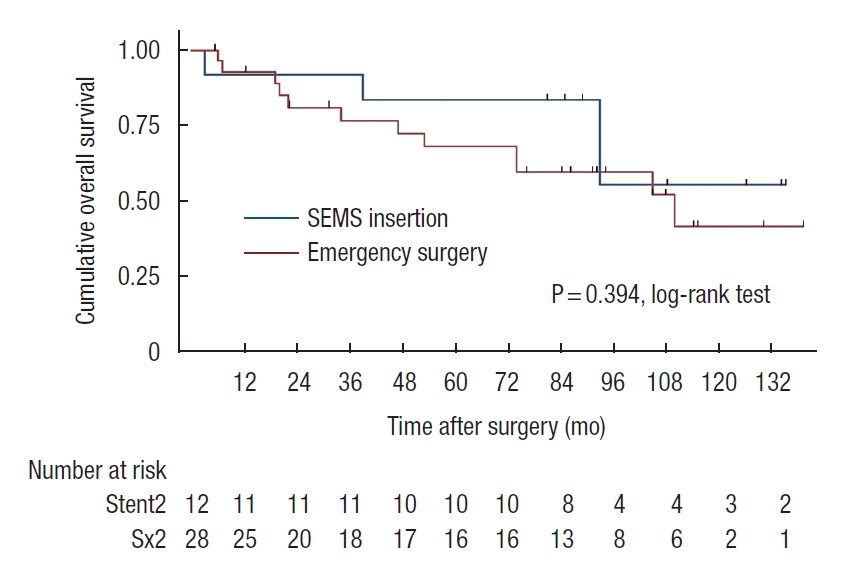
Patients who developed perforation or clinical failure after SEMS insertion were included in the SEMS group for the intent-to-treat analyses. There was a trend of worsening 5-year OS rate in SEMS group compared with emergency surgery group (45% vs. 57%, P=0.07). In stage-wise subgroup analyses, 5-year OS rates were not significantly different for stage II and IV (stage II: 83% vs. 68%, P=0.39; stage IV: 19% vs. 38%, P=0.29; Figs. 2, 3). However, a trend of deteriorating 5-year OS rate in the SEMS group with stage III was observed (43% vs. 59%, P=0.06; Fig. 4). Five-year DFS rates were not significantly different in the SEMS and emergency surgery groups (stage II: 83% vs. 61%, P=0.27; stage III: 38% vs. 38%, P=0.27; Figs. 5, 6).
Kaplan-Meier probability of overall survival of patients with stage II malignant obstruction. SEMS, self-expandable metal stent.
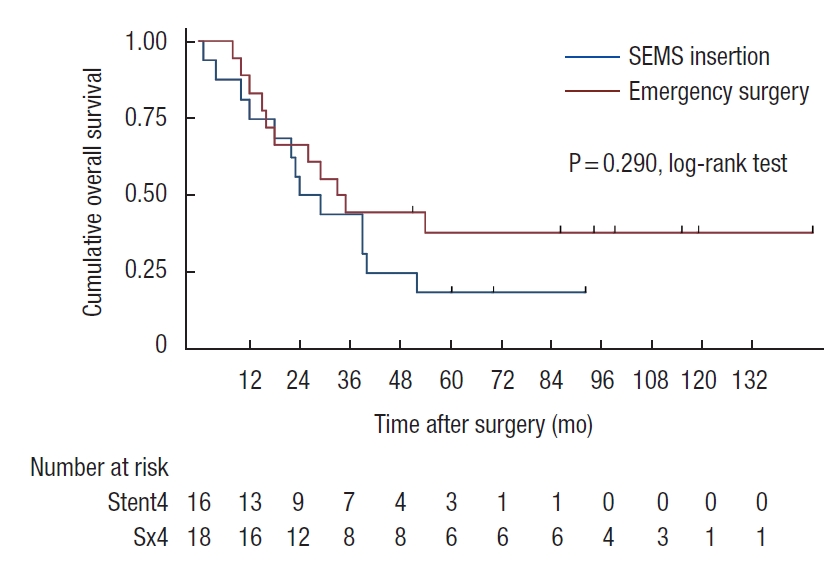
Kaplan-Meier probability of overall survival of patients with stage IV malignant obstruction. SEMS, self-expandable metal stent.

Kaplan-Meier probability of overall survival of patients with stage III malignant obstruction. SEMS, self-expandable metal stent.

Kaplan-Meier probability of disease-free survival of patients with stage II malignant obstruction. SEMS, self-expandable metal stent.

Kaplan-Meier probability of disease-free survival of patients with stage III malignant obstruction. SEMS, self-expandable metal stent.
Table 2 shows the rate and pattern of recurrence in both groups. There was no difference in the rate or site of recurrence between the SEMS and emergency surgery groups (P=0.71) for all patients. In the subgroup analysis of patients without metastases at the time of diagnosis, no differences in the rate and pattern of recurrence were observed (P=0.82).
Secondary outcomes
Table 3 shows the perioperative outcomes of both groups. In the SEMS group, the technical success rate was 100%, and the clinical success rate was 95.9%. Patients who did not improve after SEMS insertion underwent emergency surgery. The median interval to surgery after SEMS placement was 15 days (IQR, 8–29 days).
Patients in the SEMS group were more likely to undergo laparoscopic surgery than those in the emergency surgery group (51% vs. 9%, P<0.001). The SEMS group had a higher rate of primary anastomosis without stoma (71% vs. 52%, P=0.03), and a lower permanent stoma rate (8% vs. 25%, P=0.02) than the emergency surgery group. The temporary stoma rate did not differ between the 2 groups (P=0.70).
One of the 49 patients (2.0%) in the SEMS group had SEMS-related perforation that necessitated emergency colectomy. One patient (2.0%) had stent migration 10 days after insertion and underwent emergency colectomy due to symptoms of obstruction. Postsurgical complication rates were not significantly different between the 2 groups (29% vs. 44%, P=0.08). All postoperative complications were categorized using the Clavien-Dindo classification, and we found no difference in their incidence in all grades. The median length of stay after surgery was shorter in the SEMS group (8 days vs. 13 days, P=0.01) than in the emergency surgery group. No patient died within 30 days of admission.
Multivariate analyses showed that age of ≥ 65 years, American Joint Committee on Cancer stage of ≥ III, and synchronous metastasis were associated with poor OS, while adjuvant chemotherapy was associated with improved OS (Table 4). The type of intervention (SEMS insertion vs. emergency surgery) was not a prognostic factor for OS (P=0.47).
DISCUSSION
We found that SEMS as a bridge to surgery may have adverse oncologic outcomes for patients with LMCO compared with emergency surgery. Patients in the SEMS group had a trend of worsening 5-year OS rate without higher recurrence. With regard to short-term outcomes, SEMS as a bridge to surgery had a higher rate of primary anastomosis without stoma and a lower risk of permanent stoma creation.
The role of SEMS as a bridge to surgery has been debated because of concerns of negative oncologic outcomes. SEMS-related perforation has been found to impair oncologic outcomes [25]. In the Dutch Stent-in 2 trial, Sloothaak et al. [14] found that 5 out of 6 patients with perforation experience recurrence. Moreover, Gorissen et al. [15] reported that local recurrence was more common in patients who underwent SEMS insertion than in those who underwent emergency surgery (32% vs. 8%, P=0.04). The perforation rate was 8%, and all patients with perforation experienced recurrence. Sensitivity analyses showed that 3-year OS was significantly better in studies with perforation rates of < 8% than in those with perforation rates of ≥ 8% [26]. Several studies that reported low perforation rates showed comparable oncologic outcomes between SEMS as a bridge to surgery and emergency surgery [7, 17, 27, 28]. In the present study, 2% of patients experienced SEMS-related perforation that needed emergency resection. The OS rate of the patients in the SEMS group tended to be poorer than that in the emergency surgery group. By contrast, the DFS and the recurrence rates were not different. However, there were 2 major confounders of survival analysis in this study. The patients in SEMS group were older and more likely to receive the oxaliplatin-based chemotherapy with or without antiangiogenic drug. Thus, we cannot conclude from our results that SEMS insertion does not adversely affect the oncologic outcomes.
SEMS insertion may impair oncologic outcomes even in the absence of perforation. The SEMS can induce shear forces on the tumor, which can lead to the dissemination of cancer cells into the peritoneal cavity, lymphatic fluid, and bloodstream [29]. Maruthachalam et al. [12] observed a more significant increase in cytokeratin 20 messenger RNA expression in peripheral blood after SEMS insertion than after staging colonoscopy. Kim et al. [30] found that the perineural invasion rate, but not the survival rate, increased after SEMS insertion. Moreover, Yamashita et al. [13] reported tumor cell dissemination into peripheral circulation after SEMS insertion. Our study demonstrated a decreasing trend in the OS rate for SEMS as a bridge to surgery, although we observed a low perforation rate. However, it was difficult to ascertain whether tumor dissemination caused impaired OS in the SEMS group because both locoregional and distant recurrence were not higher. To date, although evidence of dissemination of tumor cells during SEMS insertion exists, there is insufficient evidence of its adverse effects on long-term survival and prognosis.
We demonstrated that SEMS insertion has high technical and clinical success rates (100% and 95.9%, respectively), with a 2% chance of SEMS-related perforation. Consequently, we obtained favorable short-term outcomes with low permanent stoma rates and high rates of primary anastomosis without stoma. Moreover, patients in the SEMS group tended to have lower rates of postoperative complications than those in the emergency surgery group (29% vs. 44%, P=0.08). To improve the short-term outcome of SEMS insertion, the technical and clinical failure rates should be minimized. In a previous study, Cheung et al. [8] reported high technical and clinical success rates (100% and 83%, respectively) with no incidence of perforation. Patients in the SEMS group had significantly lower morbidity than those in the emergency surgery group (8% vs. 70%, respectively). In a multicenter randomized trial, van Hooft et al. [31] reported a technical success rate of 70%, clinical success rate of 70%, and perforation rate of 12.7%. Morbidity tended to be higher in the SEMS group than in the emergency surgery group (53% vs. 45%, P=0.43). Thus, high success rates and low perforation rates are key factors for obtaining promising short-term outcomes following SEMS insertion.
In 2014, the European Society of Gastrointestinal Endoscopy (ESGE) guidelines did not recommend using SEMS as a bridge to surgery based on studies with low success rates and high complication rates [32]. However, many comparative studies and 1 randomized controlled trial were subsequently published. They reported high success rates and good oncologic outcomes [17-23]. Considering this, the updated ESGE guidelines released in 2020 consider SEMS insertion as a bridge to surgery as a valid treatment option in patients with LMCO. However, the risk and benefit of SEMS insertion should be discussed by the medical team. Furthermore, they underlined that SEMS insertion should be performed or directly supervised by a competent endoscopist [33].
Our study has several limitations. First, since this was a retrospective cohort study, selection bias by the consultant colorectal surgeon occurred regarding treatment choice. Older adults with comorbidities were more likely to undergo SEMS insertion as a bridge to surgery than emergency surgery. Therefore, the OS rate was confounded by the difference in age between the groups. Besides, we could not avoid the impact of the patients’ financial status and medical reimbursement on treatment selection. Patients with the Civil Servant Medical Benefit Scheme were more likely to receive SEMS insertion and oxaliplatin-based chemotherapy with or without antiangiogenic drug. The results of this study are difficult to verify because of these major confounders. Second, emergency surgery encompasses various procedures (Hartmann procedure, segmental colectomy with/without on-table lavage, subtotal/total colectomy). Each procedure has advantages and disadvantages that might affect the outcome. However, there is no clear evidence favoring 1 procedure over another. Finally, the sample size was small because this was a single-center study. This might be the reason for the differences in the OS rate not being statistically significant. However, it is easier to standardize the SEMS insertion technique in a single-center study than in a multicenter study. This might have contributed to the high success rate of SEMS insertion in our study.
SEMS as a bridge to surgery may have adverse oncologic outcomes. Patients in the SEMS group showed a trend of worsening 5-year OS rate without higher recurrence. However, the patients that underwent SEMS as a bridge to surgery were older and more likely to receive the oxaliplatin-based chemotherapy with or without antiangiogenic drug than those who underwent emergency surgery. These factors may have confounded the outcomes of this study. Therefore, further studies are needed to investigate the role of SEMS as a bridge to surgery.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.