A case report of a colouterine fistula treatment: when the patient chooses the steeplechase
Article information
Abstract
Colouterine fistula is a rare disease that is primarily treated using surgical approaches. Although invasive surgery is controversial in terms of techniques and results, minimally invasive endoscopic treatments have not been widely described. However, because it is rare for these fistulas to close spontaneously, surgical treatment is often mandatory. Appropriate management of colouterine fistula is complicated, especially when the patient refuses surgery. In this case study, we provide the first description of a minimally invasive endoscopic treatment of an iatrogenic colouterine fistula using a self-expandable metallic stent after an over-the-scope clip malposition.
INTRODUCTION
Colouterine fistula is an extremely rare disease [1–4], the treatment of which can be challenging [5, 6]. There are many possible causes of the disease, including uterine iatrogenic trauma, inflammation with abscess, uterine or sigmoidal carcinoma, and radiotherapy complications [1]. Diverticular disease (DD), complicated with diverticulitis through the rupture of a diverticulum or abscess into an adjacent organ, could be another cause [2]. Most patients who experience diverticulitis do not require surgery, but in some cases, a fistula can their worsen prognosis. The most common fistulas in DD are colovesical [3] and colocutaneous, followed by colouterine, coloenteric, or colovaginal fistulas [1]; the first treatment choice is conservative therapy [4]. However, because it is rare for these fistulas to close spontaneously, surgical treatment is often mandatory [7, 8], frequently with poor outcomes. In some selected cases, minimally invasive treatments can find some areas of use [9, 10]. We present a case of a patient affected by a concomitant diverticular stricture with a posthysteroscopy, iatrogenic colouterine fistula treated by endoscopic stenting.
CASE REPORT
A 62-year-old woman came under observation in our proctological surgery unit for gas, purulent vaginal discharge, and passage of liquid stools, especially during periods of constipation. No fever, signs of inflammation, or pain were reported. These symptoms began a week after a diagnostic hysteroscopy; during the exam, no abnormalities were reported, and there was no need for operational procedures. In her anamnesis, the patient referred to the presence of an umbilical hernia and the prescription of aspirin (100 mg) for a left carotid stenosis (<35%). She began to experience menopause 10 years before and had had a natural childbirth 40 years before that.
The patient underwent a proctologic and vaginal examination, which yielded no evidence. None of the gynecologists agreed to invasive exams through the vagina, so with suspicion of a rectovaginal fistula, magnetic resonance imaging was performed without a clear identification (Fig. 1). A contrast barium enema put in evidence an obscure tract at the connection between the rectum and upper vagina.
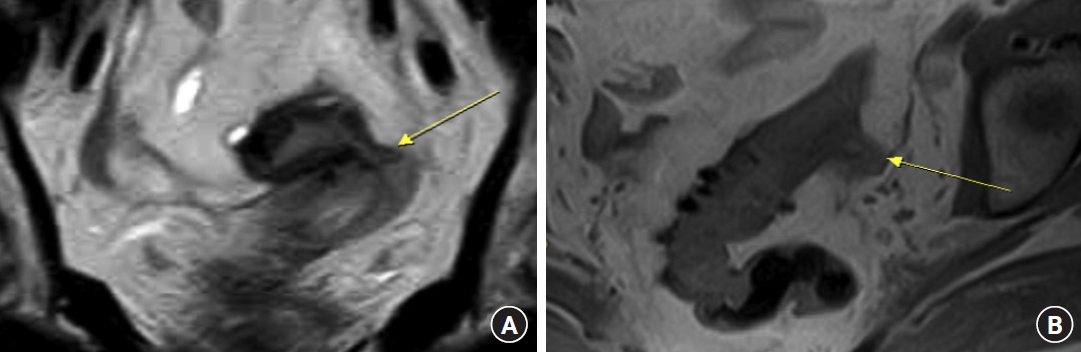
Coronal (A) and axial (B) T2-weighted magnetic resonance images (MRIs) showing sigmoid colon diverticula with perivisceral fat thickening and the likely presence of fistula within the uterus (arrows). (B) Sagittal T1-weighted MRI showing a close relationship between the sigmoid colon and uterus.
Fearing a diverting ostomy, the patient refused an invasive surgical approach for treating the initial diagnosed disease of a rectal vaginal fistula. Colonoscopy was performed to confirm the colovaginal fistula diagnosis and to choose the best minimally invasive treatment. The examination did not allow the identification of a rectal orifice, and the methylene blue test did not successfully show the passage of dye through the vagina. However, at the sigmoid level, severe diverticulosis with the presence of marked stenosis was revealed.
A second radiologically assisted colonoscopy was performed. We assumed that the orifice was in the sigmoid colon, the area most affected by DD, and we planned to treat it by positioning an over-the-scope clip (OTSC; Ovesco Endoscopy). The radiological evaluation confirmed the presence of a colouterine fistula upstream of the substenotic tract (Fig. 2). According to the plan, positioning of the OTSC was carried out. The positioning was radiologically assisted through the introduction of a guidewire and an angiographic catheter through the uterine orifice to highlight and stabilize the colic orifice in the context of multiple diverticular orifices.
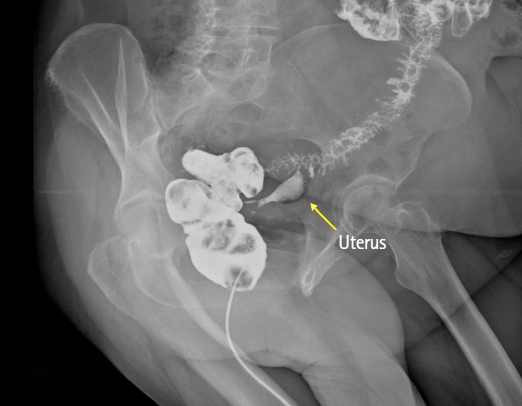
An x-ray image with iodine enema confirming a fistula with late opacification of the lumen in the uterus and stenosis of the sigmoid colon.
Unfortunately, at subsequent radiological checks, the position of the OTSC was not correct. Thus, considering the difficulty in visualizing the orifice and crossing the substenotic tract with the device mounted on a gastroscope, we chose to treat the patient by stenting [11]. A fully covered self-expandable metallic stent (SEMS; Micro-Tech intestinal covered stent; diameter 24–18 mm, length 80 mm; Micro-Tech), partially secured by 2 metallic clips, was used (Fig. 3).
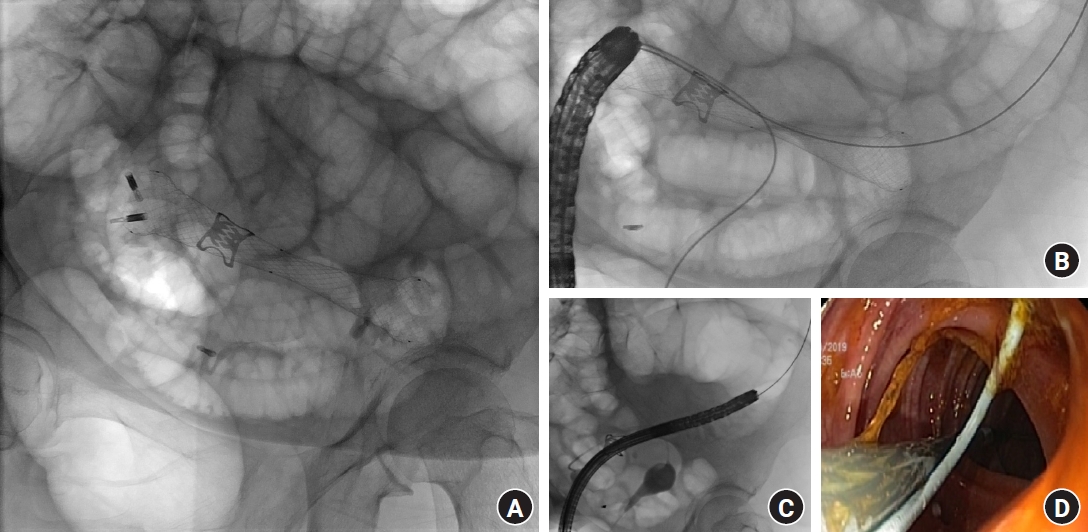
(A) Endoprosthesis was deployed to exclude the orifice of the fistula and to dilate the stenosis. (B) The endoprosthesis was fixed using metallic clips to avoid stent migration. (C) Through a transuterine retrograde approach under fluoroscopic guidance, an angiographic catheter was advanced into the colon through the fistula to locate the orifice and allow for the deployment of the endoprosthesis. (D) Endoscopic view during self-expandable metallic stent positioning.
On the 1st and 3rd days after the procedure, an x-ray was performed to confirm and monitor the correct expansion of the stent (Fig. 4). The patient did not report any symptoms. Blood exams showed initial leucocytosis (12,000 cells/mL), which disappeared after 1 day.
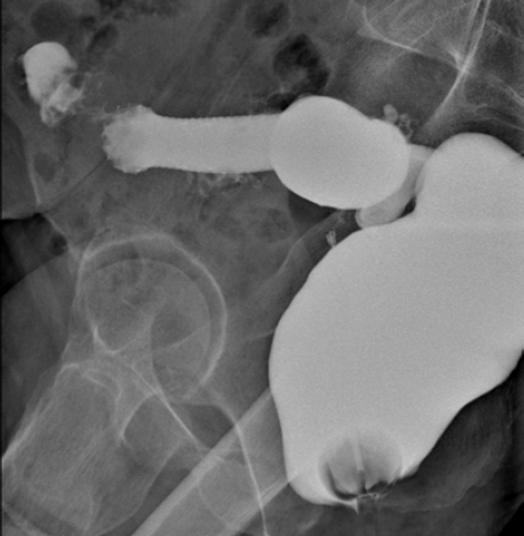
Three days after self-expandable metallic stent positioning, an iodine enema showed the correct position and patency of the stent.
The patient was discharged after 7 days with cyclic therapy for DD (rifaximin of 200 mg, 2 capsules twice a day for 5 days on the 1st week of every month), stool softeners and mesalazine (1,200 mg, 2 capsules a day) for 1 month, and the indication for a light slag-free diet. We clinically checked the patient after 1 week and after 1 month, and we scheduled a contrast enema after 3 months to plan endoscopic remotion if the treatment had been successful [10, 11]. One week before the scheduled exam, the patient complained of severe constipation, which was resolved after 3 days with a prescription of lactulose; the patient then described a foreign body in the feces. During the subsequent enema, it was evident that while the patient was constipated, the prosthesis had been expelled along with the OTSC clip. The fistula was no longer radiologically evident, and the stenosis was still present, but the contrast indicated a clear passage in the colon (Fig. 5).
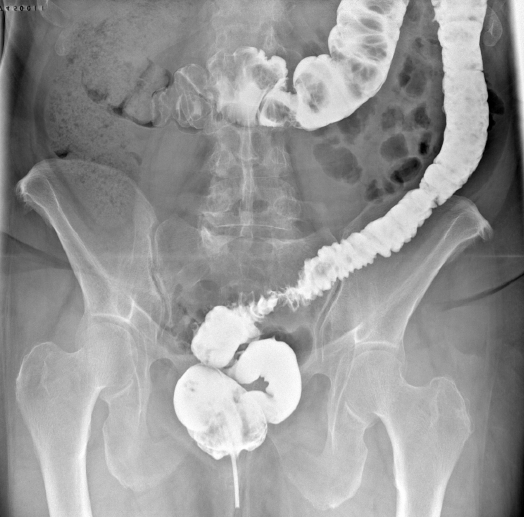
Barium enema performed 2 months after self-expandable metallic stent, after spontaneous expulsion of the endoprosthesis and clips, showed no further evidence of the colouterine fistula and partial stenosis dilation.
Ethics statement
Written informed consent was obtained for publication of this case report and accompanying images.
DISCUSSION
The current therapy for DD when complicated with a fistula is surgery, but this can be very challenging to perform, particularly in rare cases of a fistula with a connection between the bowels and uterus [10]. In one of the most extensive experiments on this issue in the literature, DeLeon et al. [2] reported on 52 women who were surgically treated for colovaginal fistulas with a good percentage of success (90%); a diverting ostomy was conducted in 28 of the patients (54%), and the authors reported the need for a second operation to address failed fistula closure attempts in 5 patients and the placement of ureteral stents in 25 patients (50%).
The presence of inflammation caused by DD and abscesses can distort the intended dissection plan during surgery. For this reason, even in the presence of an experienced surgeon, a diverting ostomy is often mandatory to protect a sigmoidectomy or a hysterectomy [7]. Because of our patient’s refusal of a major operation or diverting ostomy, we considered the alternative treatment of clipping or stenting.
OTSCs represent a valid alternative to surgery in the case of fistulas in the digestive tract. However, in some cases, the technical limitations of the device may result in an imperfect closure [12]. In our case, 2 factors contributed to the incorrect OTSC positioning—stiffness of the bowel because of the presence of inflammation and diverticular stenosis, with difficult aspiration/grasping of the defect inside the cap where OTSC was placed, and the imperfect visualization of the orifice between 2 colic folds.
Although it has been reported that OTSC removal is conducted by cutting at 2 opposing sites using a bipolar grasping device to apply short direct current impulses, we chose not to perform this procedure in a section of the colon affected by diverticular stenosis. Thus, although SEMS use was initially postponed due to a high rate of complication when placed in a patient with a severe DD affected by stenosis [13–15], we considered this treatment as a second step to be done without removing the OTSC.
Our case represents the first description of a minimally invasive endoscopic treatment of an iatrogenic colouterine fistula by SEMS. Furthermore, we have shown that when surgery is refused, the positioning of a stent can also be considered in unfavorable conditions, such as the presence of an OTSC and a complicated DD.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: SP, DM; Data curation: CE; Investigation: SP, PGN, DM; Supervision: SP; Writing–original draft: CE; Writing–review & editing: all authors. All authors read and approved the final manuscript.
