Preoperative endoscopic tattoo marking improves lymph node retrieval in laparoscopic rectal resection: a retrospective cohort study
Article information
Abstract
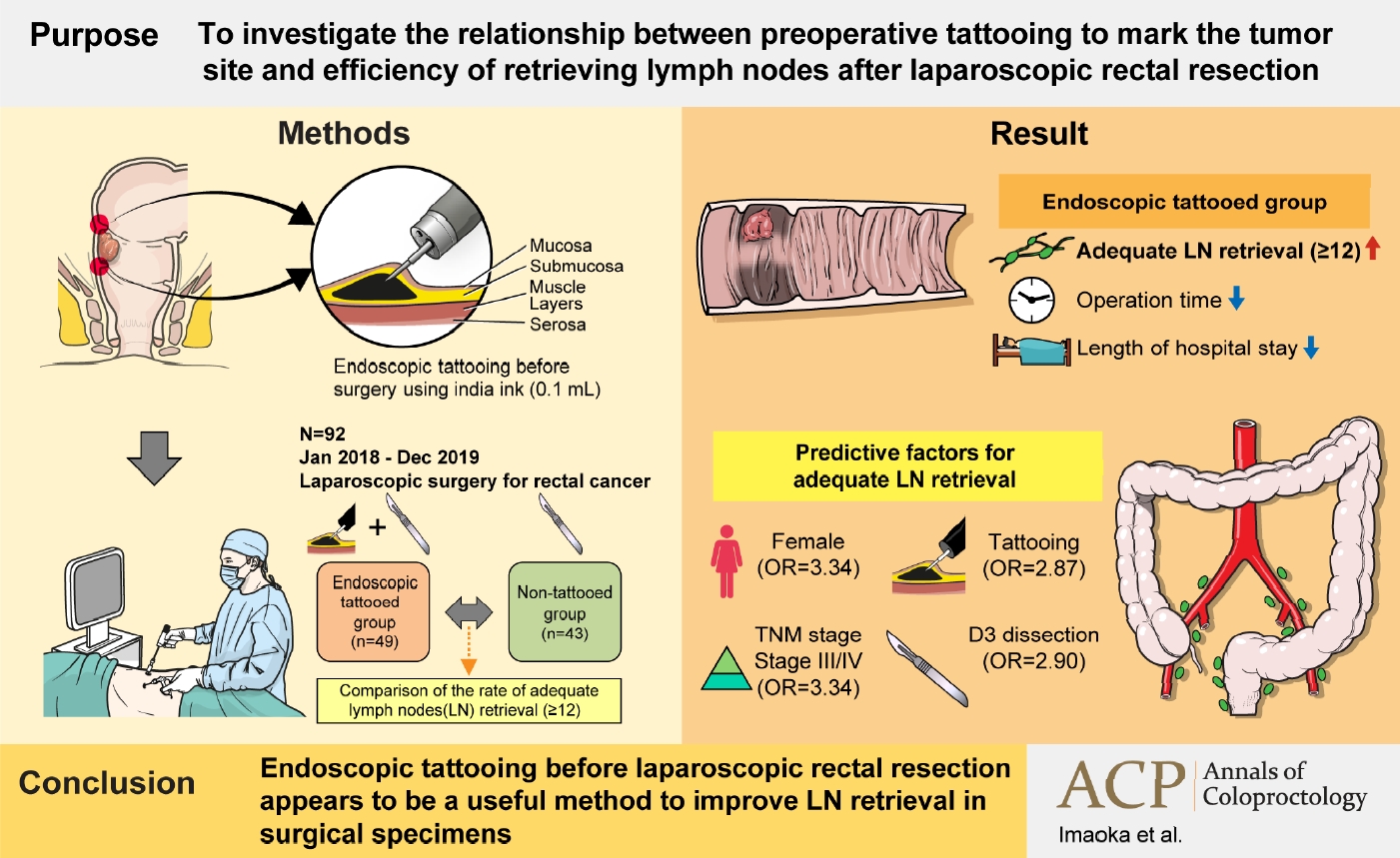
Purpose
Harvesting at least 12 lymph nodes (LNs) is recommended for adequate tumor staging in colon surgery. Although preoperative endoscopic tattooing has been used for primary localization of tumors, its impact on LN retrieval in colorectal surgery remains controversial. We aimed to investigate the relationship between preoperative tattooing and LN retrieval after laparoscopic rectal resection.
Methods
We reviewed the records of 92 patients with rectal cancer who underwent laparoscopic resection from January 1, 2018 to December 31, 2019. Patients were categorized into 2 groups according to whether preoperative endoscopic tattooing was performed. The rate of adequate LN retrieval (≥12) was compared.
Results
The tattooed and non-tattooed groups comprised 49 and 43 patients, respectively. In the tattooed and non-tattooed groups, the rates of adequate LN retrieval were 75.5% and 55.8%, respectively (P=0.046). Univariate analysis revealed that female sex, tattooing, LN metastasis status, pathological pathological stage (p-stage), and LN dissection were predictive factors for adequate LN retrieval. In the multivariate analysis, female sex (odds ratio [OR], 3.34; 95% confidence interval [CI], 1.15–9.73; P=0.027), tattooing (OR, 2.87; 95% CI, 1.03–7.94; P=0.043), and p-stage (OR, 3.34; 95% CI, 1.04–10.75; P=0.043) were independent predictive factors for adequate LN retrieval after surgery.
Conclusion
This study revealed that preoperative endoscopic tattooing was statistically significantly associated with adequate LN retrieval in patients with rectal cancer who underwent laparoscopic rectal resection. Preoperative endoscopic tattooing should be considered to improve disease assessment and avoid stage migration.
Graphical Abstract
INTRODUCTION
A direct correlation has been previously reported between the number of lymph nodes (LNs) retrieved after colectomy for cancer and patient survival [1]. The American Joint Committee on Cancer, Union for International Cancer Control, and Japanese Society for Cancer of the Colon and Rectum (JSCCR) recommend harvesting at least 12 LNs for adequate tumor staging [2, 3]. Patients with a node-negative disease or node-positive disease in less than 12 LN specimens are considered suitable for adjuvant chemotherapy [4]. However, many factors can affect LN retrieval in colorectal cancer specimens. In this regard, the surgical technique and pathological analysis are the foundations for adequate LN retrieval and staging. For example, complete mesocolic resection and central venous ligation may potentially yield more LNs by removing a large amount of tissue surrounding the tumor [5]. The nonsurgical factors that can impede LN retrieval include older age, obesity, and male sex. In contrast, right-sided, large, and poorly differentiated tumors are associated with an increased number of LNs retrieved [6].
Preoperative endoscopic tattooing has been used to localize colorectal tumors. However, the impact of preoperative tumor tattooing on LN retrieval is controversial [7–12]. Moreover, the site of the tumor significantly influences the efficiency of LN retrieval. Tumors located in the rectum tend to yield fewer LNs than those in the colon [13]. Given that retrieving a sufficient number of LNs in rectal cancer surgery remains a challenge for surgeons, new strategies are required to improve the accuracy of the technique for harvesting LNs. Therefore, this study aimed to investigate the relationship between tattooing to mark the tumor site and efficiency of retrieving LNs after laparoscopic rectal resection. To the best of our knowledge, this is the first large retrospective study that investigated the impact of preoperative tattooing in patients with rectal cancer undergoing laparoscopic rectal resection and LN retrieval.
METHODS
Ethics statements
This study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and was approved by the Institutional Review Board of Hiroshima City Hiroshima Citizens Hospital on July 3, 2020 (No. 2020-45). The requirement for informed consent was waived because of the retrospective nature of this study.
Eligibility
We included patients who underwent laparoscopic surgery for rectal cancer at our institution from January 1, 2018 to December 31, 2019. The clinical data, including the baseline patient characteristics, tumor-specific characteristics, pathological tumor staging, operative findings and methods, morbidity, duration of hospital stay, and number of harvested nodes, were collected retrospectively from medical records. The control group comprised non-tattooed patients who underwent an elective resection for rectal cancer during the same period at the same hospital. The exclusion criteria were multiple colorectal carcinomas and laparoscopic rectal resections with insufficient D0 or D1 LN dissection. Lateral LN dissections were also excluded from analysis because the dissection area was added and the number of dissected LNs changed, and it was not clear whether the tattoo would migrate to this lateral area. Furthermore, patients who underwent preoperative chemoradiation therapy (pre-CRT) were excluded, as this could have resulted in fewer LNs harvested during surgery.
Colonoscopy and tattooing
The decision to perform preoperative endoscopic tattooing depended upon the surgeon. Preoperative tattooing was sometimes omitted if the tumor was proximal to the anal verge or identified with intraoperative colonoscopy. The tumor was localized during a colonoscopy and marked with ink for tattooing by a gastroenterologist before surgery. An injection needle delivered ink through the working channel of the endoscope. The injection needle was positioned in the submucosa and connected to a syringe containing sterilized India ink. India ink (0.1 mL) was injected, and the needle was flushed with saline solution. The tattoos were placed on the ventral side of the lumen and anal side of the tumor.
Surgical technique
Laparoscopic rectal resection was performed under general anesthesia. For each patient, rectal resection was performed following oncological criteria comprising proximal vessel ligation, en bloc lymphadenectomy, and resection with broad macroscopic margins according to the total mesorectal excision (TME) principle. According to the Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma, the optimal length of bowel resection is determined such that the pericolic/perirectal LN is dissected [14]. According to the JSCCR guidelines, we determined the extent of the pericolic/perirectal LNs in each case of rectal cancer. The oral side was determined by the lowest plunge point of the sigmoid artery, while the anal side was determined by a distance of at least 3 cm from the tumor margin in the rectosigmoid and the upper rectum/above the peritoneal reflection cancers and 2 cm in the lower rectum/below the peritoneal reflection cancers [3]. The extent of LN dissection was determined based on the preoperative clinical findings, extent of LN metastasis, and depth of tumor invasion observed intraoperatively, according to the JSCCR guidelines [3]. A D3 dissection was performed if LN metastasis was recognized or suspected based on the preoperative or intraoperative findings. TME and lateral LN dissection surgery without pre-CRT has been the standard strategy for advanced rectal cancer in the JSCCR guidelines, while in many countries, pre-CRT has long been a part of the standard treatment for rectal cancer [3]. A lateral LN dissection was performed when (a) the lower border of the tumor was located distally to the peritoneal reflection, (b) the tumor invaded beyond the muscularis propria, and/or (c) lateral LN metastasis was suspected based on the preoperative findings. Neoadjuvant concurrent chemoradiotherapy was chosen first if a circumferential resection margin ≥ 1 mm, an indication for radical surgery for locally advanced rectal cancer, could not be confirmed. The surgeon decided whether the left colic artery would be preserved depending on the patient’s condition.
Pathological examination
According to standardized protocols, all mesenteric tissues were manually examined for LNs by 2 surgeons before formalin fixation. The surgeons identified the LNs by sight and palpation, and the black dye in the LNs was easily visible. The intraoperative appearance of carbon-containing LNs is shown in Fig. 1. Earlier studies have already revealed the intraoperative, pathological, and histological appearance of carbon-containing LNs, which can be used as references [7, 8]. All extracted LNs were stored in glass containers. Chemical fat clearing techniques were not used. Two pathologists performed the pathological examinations. The staging and grading of the tumor were performed according to the JSCCR guidelines [3]. The LN metastasis status was evaluated via histopathological examination; hence, the LN metastasis status is a type of pathological lymphatic staging. The LN metastasis status was defined as “yes” when LN metastasis was positive pathologically, otherwise “no.”
Outcome measures
This study’s primary outcome was the rate of adequate LN retrieval per specimen. The postoperative complications were categorized according to the Clavien-Dindo classification system [15]. Postoperative mortality and morbidity were defined as death from any cause and any adverse event of grade ≥ 1, including anastomotic leakage, ileus, surgical site infection, and pneumonia, within 30 days after surgery.
Statistical analyses
Continuous data are presented as medians with ranges (minimum–maximum). Categorical data are presented as frequencies and percentages. Data from the 2 groups were compared using the chi-square test or Fisher exact test for dichotomous outcomes. The Mann-Whitney U-test was used for continuous outcomes with a skewed distribution. Univariate analyses were performed to assess the association of adequate LN retrieval with all variables: age, sex, body mass index, tattooing, tumor location, histological type, LN metastasis status, pathological stage (p-stage), LN dissection, and left colic artery preservation. All variables were included in the multivariate logistic regression model by using a stepwise (forward/backward) procedure. Independent variables were entered into the model at the 0.10 significance level and removed at the 0.20 level, and these were used to select covariates. A P-value of < 0.05 was considered statistically significant. Statistical analyses were performed using JMP statistical software ver. 14 (SAS Institute Inc).
RESULTS
A total of 92 patients with rectal cancer were included in this study and categorized into 2 groups: 49 who underwent preoperative endoscopic tattooing and 43 who did not (the control group, “non-tattooed”). The baseline characteristics, tumor-specific characteristics, and pathological tumor staging in the tattooed and non-tattooed groups are presented in Table 1. There was no difference in the age, sex ratio, or body mass index between the 2 groups. Most clinical and pathological parameters, such as T and N categories, LN metastasis status, and p-stage, were comparable between the 2 groups. However, the tumor location was significantly different. A higher percentage of tumors was located above the peritoneal reflection in the tattooed group than in the non-tattooed group (P=0.001). The histological type of the tumors was also significantly different. Tubular or papillary adenocarcinoma was more common in the tattooed group than in the non-tattooed group (P=0.044).
Table 2 shows the operative findings and methods, morbidity, and duration of hospital stay in both groups. There were no significant differences in the extent of LN dissection, preservation of the left colic artery, blood loss, or overall postoperative complications between the 2 groups. However, there was a significant difference in the choice of operative method. A high anterior resection was frequently performed in the tattooed group. In contrast, a low anterior or super low anterior resection was frequently performed in the non-tattooed group. In addition, the percentage of covering stomas was significantly higher in the non-tattooed group. Moreover, the operative time and hospital stay were significantly longer in the non-tattooed group than in the tattooed group. In the tattooed and non-tattooed groups, the rates of adequate LN retrieval were 75.5% and 55.8%, respectively (P=0.046).
Adequate LN retrieval was achieved in 61 patients (66.3%). Univariate analysis revealed that sex, tattooing, LN metastasis status, p-stage, and LN dissection were predictive factors for adequate LN retrieval. Multivariate analysis revealed that female sex (odds ratio [OR], 3.34; 95% confidence interval [CI], 1.15–9.73; P=0.027), tattooing (OR, 2.87; 95% CI, 1.03–7.94; P=0.043), and p-stage (OR, 3.34; 95% CI, 1.04–10.75; P=0.043) were independent predictive factors for adequate LN retrieval after laparoscopic rectal surgery (Table 3).
DISCUSSION
This study revealed that routine preoperative endoscopic tattooing, female sex, and p-stage were significantly associated with adequate LN retrieval in patients with rectal cancer who underwent laparoscopic rectal resection.
Adequate LN sampling has been frequently regarded as an indicator of oncological clearance and quality of cancer surgery [16, 17]. Studies have shown that higher numbers of analyzed LNs are related to longer survival among patients with colon cancer [18–20]. This might be because of the stage migration effect, where an increased number of retrieved LNs leads to an increased upgrade of patients to stage III disease [21].
LN retrieval is affected by multiple factors. Studies have shown that a higher LN retrieval is associated with the following non-modifiable factors: patient factors, including young age, female sex, and ethnicity, and tumor factors, including advanced T and N stages, right-sided tumors, greater tumor size, and poorly differentiated tumors [6, 22–24]. Both female sex and p-stage were independent predictive factors for adequate LN retrieval in our study, which was consistent with that the results in those previous studies. Among these non-modifiable factors, the relationship between tumor location and LN retrieval is extremely important. In a retrospective study, Wang et al. [13] reported that LN retrieval was significantly lower in patients with rectal carcinoma than in those with colon carcinoma. In addition, Chou et al. [22] showed that tumors located in the right colon tended to yield a greater number of LNs than sigmoid, rectosigmoid, and rectal tumors. The feeding arteries of colon cancers depend upon the tumor’s location, while the tumor’s location may also influence the range of the resection. In this regard, we speculate that a resected specimen from the right colon should be longer than one from the left colon. However, the location of rectal cancer has little influence on the extent of the resection and length of specimens because the feeding artery is the inferior mesenteric artery. Since right-sided colon surgery requires the ligation of multiple vessels, including the ileocolic, right, and middle colic arteries, the dissection range is greater than that in rectal surgeries, where the inferior mesenteric artery alone needs to be ligated. The factors described above may be responsible for the reduced LN retrieval observed during rectal cancer resection. In terms of LN retrieval, differentiating rectal cancers from colon cancers is necessary. Our study focused solely on rectal cancers. Pre-CRT followed by curative resection is the standard practice for most patients with locally advanced rectal cancer, considering the risks of recurrence and mesorectal LN involvement [25]. However, pre-CRT has recently been reported to reduce the number of retrieved LNs [22, 26]. Therefore, we excluded cases with pre-CRT from analysis in this study.
Preoperative endoscopic tumor tattooing before LN retrieval remains controversial. Tattooing may be a useful method for sentinel LN mapping and retrieval [8]. Kang et al. [27] demonstrated that preoperative tattooing was associated with a higher LN yield in 61 patients with T1 rectal cancer (8 in the tattooed group vs. 53 in the non-tattooed group). Previous studies have examined the impact of preoperative tattooing on LN retrieval in both colon and rectal cancer specimens [7–10]. However, only a few reports have focused solely on rectal cancers, showing that preoperative endoscopic tattooing improved LN retrieval for patients with rectal cancer receiving neoadjuvant concurrent chemoradiotherapy [28].
It has been shown that carbon particles from the tattoo ink are engulfed by macrophages and deposited in the sinuses of the LNs [29]. The deposition of carbon in LNs can cause grossly visible pigmentation that can be easily identified by both the surgeon and pathologist. The intraoperative appearance of carbon-containing LNs is shown in Fig. 1. Although tattoo ink does not increase the number of LNs harvested during surgery, it prevents LNs in the specimen from being missed and results in improving the LN retrieval rate in the pathological process.
Endoscopic tattooing was originally developed to facilitate tumor localization during laparoscopic resection, and it is currently the safest and most effective method for localizing colorectal lesions, especially small lesions, flat tumors, or those at polypectomy sites [11]. Clinically relevant tattooing complications are considered rare; the rate of India ink leakage into the peritoneal cavity was reported to be 1.8% [30, 31]. Leakage of ink into the peritoneal cavity can cause severe adhesion and inflammation, making it difficult to perform a safe surgical resection, particularly during laparoscopic surgery. This study revealed that preoperative tattooing did not influence blood loss or postoperative complications when compared with no tattooing. Thus, preoperative endoscopic tattooing can be considered a safe and helpful method for LN resection.
This study had some limitations. First, the clinicopathological analysis was performed retrospectively for rectal cancer specimens; therefore, a bias may exist between the different groups. Second, selection bias can occur when the surgeon decides whether to perform preoperative endoscopic tattooing. Preoperative tattooing was sometimes omitted if the tumor was proximal to the anal verge or identified during intraoperative colonoscopy. Therefore, tumors in the tattooed group were located significantly farther from the anal verge than those in the non-tattooed group, resulting in the choice of different operative methods, longer operative time, and extended hospital stays in the latter group. As tumors in the non-tattooed group were closer to the anal verge, they required resection of a larger portion of the rectum, which included the lower rectum. We expected that the non-tattooed group would yield more LNs because the non-tattooed group would require a wider excision of the mesentery; however, the results were the opposite. Therefore, we believe that the operative method and tumor location between the 2 groups did not affect LN retrieval in this study. Finally, our study was performed at a single institution, and the number of cases was small. Thus, prospective studies including a higher number of patients are required for further evaluation.
In conclusion, our analysis found that routine preoperative endoscopic tattooing was significantly associated with adequate LN retrieval in patients with rectal cancer. Endoscopic tattooing before laparoscopic rectal resection appears to be a useful method to improve LN retrieval in surgical specimens and to avoid stage migration in rectal cancer.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.
AUTHOR CONTRIBUTIONS
Conceptualization: KI, TY, MY, MO; Data curation: KI, TY, MY, KN, MK, HI; Formal analysis: all authors; Visualization: KI; Writing–original draft: KI, TY, MY, MO; Writing–review & editing: all authors. All authors read and approved the final manuscript.