Pretreatment inflammatory markers predicting treatment outcomes in colorectal cancer
Article information
Abstract
We aimed to review whether pretreatment inflammatory markers reflect the short- and long-term outcomes of patients with colon cancer, rectal cancer, colon and rectal cancers, and metastatic colorectal cancer (CRC). We found that pretreatment complete blood count and blood chemistry tests reflect short-term and long-term oncological outcomes in patients with CRC. Specifically, in patients with colon cancer, hypoalbuminemia was associated with worse postoperative morbidity, mortality, and inferior survival. In patients with rectal cancer, elevated neutrophil-lymphocyte ratio (NLR) and thrombocytosis were associated with postoperative complications, poor overall survival (OS), and disease-free survival (DFS). A high C-reactive protein/albumin ratio (CAR) was associated with poor OS and DFS. In patients with metastatic CRC, increased NLR and platelet-lymphocyte ratio (PLR) were associated with poor OS, DFS, and progression-free survival (PFS). In addition, high CAR and a low albumin/globulin ratio on blood chemistry tests were associated with poor OS and PFS. Although universal cut-off values were not available, various types of pretreatment laboratory markers could be utilized as adjuncts to predict prognosis in patients with CRC.
INTRODUCTION
Colorectal cancer (CRC) is the third most common malignancy and one of the leading causes of cancer-related deaths worldwide [1]. Although remarkable developments in treatment modalities have improved the prognosis of patients with CRC, long-term survival remains poor, with a 5-year survival rate of approximately 60% in patients who undergo curative resection [2, 3]. Approximately 50% of patients will eventually develop metastases, with 25% of CRC patients having distant metastasis at their initial diagnosis [4, 5]. Therefore, it is important to identify factors associated with poor prognosis in patients with CRC. The best-known prognostic factor in patients with CRC is the TNM staging system proposed by the American Joint Committee on Cancer. In addition, tumor location and genetic information are factors related to prognosis [6]. However, patients with similar characteristics and the same disease stage may have different prognoses.
There have been a number of studies investigating several pretreatment laboratory markers that can help in diagnosing cancer and predicting short-term and long-term prognoses. Such pretreatment laboratory markers can be easily assayed in routine blood tests, and their prognostic value for various types of cancer, including CRC, has been confirmed in numerous studies [7-11]. Routine blood tests performed in medical institutions include complete blood counts (CBCs) and blood chemistry panel tests. Representative available parameters include hemoglobin, neutrophils, lymphocytes, platelets, and composite markers, such as the neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) in CBCs. In addition, C-reactive protein (CRP), albumin, and composite markers, such as the Glasgow Prognostic Score (GPS) and CRP/albumin ratio (CAR) can be assessed in blood chemistry tests.
These markers can reflect the inflammatory conditions of patients. Numerous studies have suggested that systemic inflammatory responses play an important role in carcinogenesis and cancer progression, and hematologic markers reflecting systemic inflammation can be used to predict the prognosis of cancer [12-14]. The purpose of this paper was to review the currently investigated pretreatment inflammatory markers and whether they can reflect the short- and long-term outcomes of patients with CRC.
INFLAMMATORY MARKERS
Blood tests
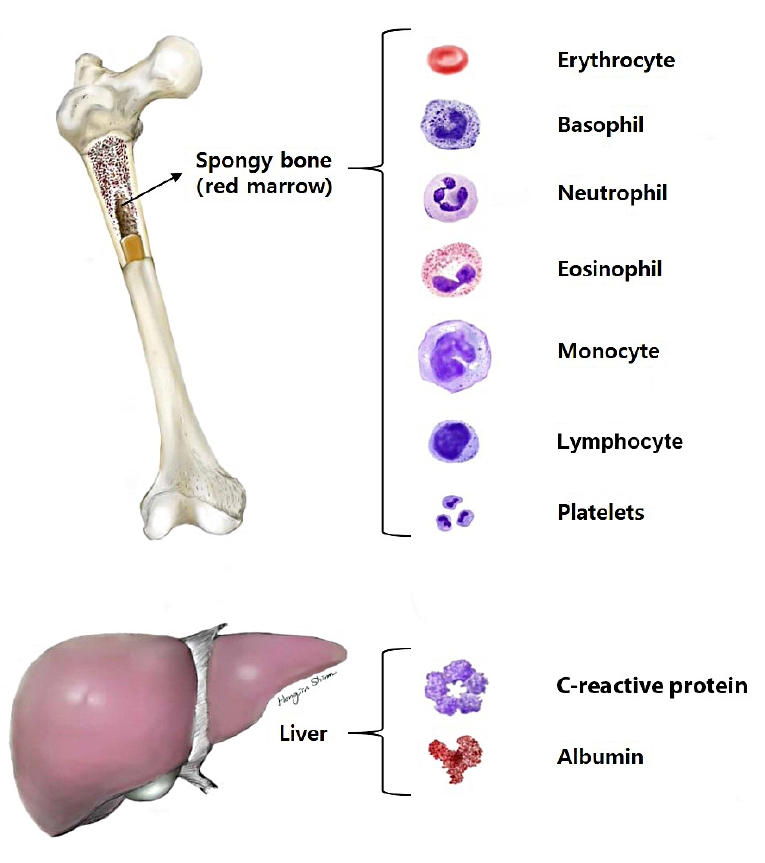
Blood is a specialized connective tissue composed of cellular components and a fluid component referred to as plasma. Cellular elements account for approximately 45% of the circulating blood volume, and plasma constitutes approximately 55%. Cells in the blood are composed of erythrocytes known as red blood cells (RBCs), granular and agranular leukocytes, also known as white blood cells (WBCs), and thrombocytes known as platelets [15].
A CBC is a laboratory test used for screening and includes measuring the numbers of WBCs, RBCs, and platelets, the concentration of hemoglobin, hematocrit, and red cell indices, such as the mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration. Blood chemistry tests include many types of chemistry panels, such as liver, renal, lipid, electrolyte, and hormonal panels. CRP and albumin levels were measured using chemistry tests (Fig. 1).
Inflammation and cancer
Chronic inflammation induces tissue damage, and cell proliferation occurs during the healing process [12, 13]. Repeated tissue damage and regeneration in chronic inflammatory situations result in permanent genetic mutations, such as point mutations, deletions, or rearrangements. Activated inflammatory cells produce chemokines and cytokines, which can influence tumor growth, migration, and differentiation by releasing growth factors, promoting angiogenesis and lymphangiogenesis, and stimulating DNA damage. Once carcinoma arises, these tumor cells turn on the expression of chemokine receptors, such as CXCR4. The production of ligands for these receptors in other organs, such as lymph nodes, bone marrow, liver, and lung, facilitates tumor migration and invasion to secondary sites. Some tumor cells, such as those in melanoma, directly regulate the expression of chemokines involved in angiogenesis, tumor growth, progression, and metastasis [16].
MARKERS AVAILABLE THROUGH A COMPLETE BLOOD COUNT
Neutrophils
Neutrophils are the most abundant WBCs in the circulation and are produced in the bone marrow daily. Neutrophils are known to play an important role in the acute inflammatory response, and in inflammatory situations, the production rate increases up to 10 times compared to normal levels. These are the first leukocytes recruited at the site of inflammation and can eradicate pathogens through several mechanisms. In addition, neutrophils are associated with numerous pathologies, including tumors and chronic inflammation [17, 18]. Neutrophils are involved in various carcinogenic processes, such as tumor initiation, growth, and proliferation, spread to other tissues, and the formation of new blood vessels in the tumor [19]. Tumor initiation can be facilitated by the release of reactive oxygen species, or proteases. Neutrophils can weaken the immune system and promote tumor growth. In addition, neutrophils can cause metastatic spread by suppressing natural killer function and promoting extravasation of tumor cells.
Lymphocytes
Lymphocytes are a subtype of WBCs. Lymphocytes develop and mature in the bone marrow and thymus, and differentiate into natural killer cells (NK cells), T-cells, and B-cells. B and T lymphocytes are involved in the adaptive immune responses, and NK cells are involved in innate immunity [20, 21]. Lymphocytes have been recognized to play a key role in combating tumor progression. A high density of lymphocytic infiltration along the invasive margin of the tumor is an independent prognostic factor for improved survival [22-24]. In contrast, immune deficiency is associated with an increased frequency of certain malignancies [25]. Immunocompromised patients, including those infected with human immunodeficiency virus and those who received transplants, were found to have a higher incidence of cancer, especially infection-related cancers [26, 27]. In addition, several studies have revealed that lymphopenia observed in advanced cancer, including CRC, correlates with unfavorable prognostic factors in terms of progression-free survival (PFS) and overall survival (OS) [28].
Platelets
Platelets are important components of the blood and are derived from megakaryocytes in the bone marrow. The primary function of platelets is to stop bleeding by adhesion and aggregation at the site of tissue and vascular injury [29]. Platelets also play important roles in host inflammation and the immune system, in general. Platelets activate leukocytes and release numerous chemoattractants and antimicrobial mediators to directly kill pathogens [30]. Platelet count is known to have an association with tumors. Tumor cells stimulate the production of thrombopoietin by secreting interleukin (IL)-6, which contributes to thrombocytosis and hypercoagulability in patients [31]. It is defined as paraneoplastic thrombocytosis, and approximately 1/3 of cancer patients show thrombocytosis at the time of diagnosis [32]. Increased and activated platelets release growth factors that facilitate tumor growth and invasion. Platelets also contribute to tumor metastasis by facilitating cancer cell adhesion and extravasation [31, 33]. Numerous studies have shown that thrombocytosis is associated with long-term prognosis in patients [34-37]. In a multicenter study of patients with advanced rectal cancer who received neoadjuvant chemoradiation (CRT), Belluco et al. [35] demonstrated that thrombocytosis before neoadjuvant CRT was associated with worse oncologic outcome. They set the platelet count cut-off value of 300×109/L by receiver operating characteristics (ROC) curve and showed thrombocytosis was associated with poor 5-year OS, disease-free survival (DFS), and higher local and distant metastasis. In addition, thrombocytosis was confirmed as an independent prognostic factor for pathologic complete response in the study.
Neutrophil-lymphocyte ratio
The literature shows that many studies have combined these inflammatory markers to predict the tumor prognosis. The NLR is a widely used combination marker, and NLR is defined as the absolute neutrophil count divided by the absolute lymphocyte count. High NLR, that is, increased neutrophil levels and decreased lymphocyte levels, have been suggested as poor prognostic markers in CRC [38-40]. Several studies have shown that a high preoperative NLR is a useful predictive marker for short-term outcomes in patients with CRC [37, 41, 42]. A study by Xia et al. [37] analyzed the correlation between various inflammatory markers and postoperative morbidity; and among them, it was found that postoperative morbidity significantly increased when the NLR was 2.8 or higher. In a study by Gohil et al. [41], in which the preoperative NLR value of 4.3 was set as the cut-off value, patients with elevated NLR are more likely to have a prolonged hospital stay after operation. In addition, one study analyzed the relationship between preoperative NLR and the amount of ileostomy after colorectal surgery. In the study, high-output ileostomy was defined when the stoma output was more than 2,000 mL/day for 3 or more consecutive days, and the optimal cut-off value of preoperative NLR was set 3.0 by ROC curve analysis. The study revealed that a high preoperative NLR is a predictor of high-output ileostomy after colorectal surgery [43]. In addition to short-term outcomes, many studies have also shown that the NLR is a good predictor of long-term oncological outcomes [44, 45]. A study by Chiang et al. [44] demonstrated that the preoperative elevated NLR (> 3) was associated with worse 5-year DFS, and another study also revealed that the preoperative elevated NLR (> 3) could reflect poor 5-year DFS and cancer-specific survival (CSS) [45]. In several studies of patients with advanced rectal cancer who received neoadjuvant CRT, the NLR was found to be a useful marker for predicting pathological tumor regression or pathologic complete response [46, 47]. One study, which set the optimal cut-off NLR value as 3, showed that elevated NLR before CRT was significant predictor for poor pathologic response [46]. However, another study by Jeon et al. [47] suggested that the elevated NLR (> 3.23) after CRT was an independent negative predictive factor for pathologic complete response, and they also showed that elevated NLR was an independent poor prognostic factor for recurrence-free survival (RFS). In studies involving patients with resectable CRC liver metastasis, pretreatment NLR has been demonstrated to be a good predictor of long-term prognosis in terms of 5-year OS and DFS [48, 49]. Moreover, several studies have reported that pretreatment NLR could reflect the effect of palliative chemotherapy in patients with unresectable metastatic CRC [50-52] (Table 1).
Platelet-lymphocyte ratio
The PLR, defined as the ratio of platelet count to lymphocyte count, has also been suggested as a prognostic marker for CRC. Elevated PLR, that is, increased platelet count and decreased lymphocyte count, have been suggested to be poor prognostic factors in CRC [53, 54]. A retrospective study with T1 to 2 rectal cancer patients by Xia et al. [37] demonstrated that a pretreatment-elevated PLR (> 140) was correlated with a higher postoperative morbidity rate. However, the association between PLR and longterm outcomes was not demonstrated in their study. In contrast, another study by Ozawa et al. [54] set the optimal cut-off value of preoperative PLR as 25.4 by ROC curve analysis and revealed that pretreatment PLR is an independent prognostic factor for long-term oncological outcomes in terms of 5-year DFS and CSS in stage II patients with CRC. Huang et al. [55] performed a meta-analysis including 17 studies with CRC patients and reported that elevated PLR was reported to be associated with poor OS, DFS, CSS, and RFS. This systematic review also concluded that elevated PLR was associated with poor clinicopathological characteristics, such as high tumor stage and poor differentiation. In addition, in a study of patients who underwent liver resection for CRC liver metastasis, preoperative PLR was an independent prognostic factor for 5-year DFS and OS. The study determined the optimal cut-off value of preoperative PLR and NLR as 150 and 2.4 by ROC curve analysis, and this study suggested that PLR is a better predictor of long-term oncologic outcomes than NLR through multivariate analysis [56] (Table 2).
MARKERS AVAILABLE THROUGH BLOOD CHEMISTRY TESTS
Albumin
Albumin is a protein that is produced from the liver and accounts for approximately 60% of the serum protein, making up the largest proportion. It is a marker that reflects nutritional condition, and its production is regulated by osmotic colloid pressure, inflammatory state, and nutritional status. Systemic inflammation reduces albumin concentration, regardless of malnutrition [57]. Malignant neoplasms exert a great deal of stress on the human body via tissue hypoxia and necrosis, causing tissue damage. To respond to these stressful changes, the human body produces various types of proinflammatory cytokines, such as IL-6, IL-1, tumor necrosis factor alpha (TNF-α), and growth factors. Consequently, hepatocytes increase the production of acute-phase proteins, such as CRP, and decrease the production of albumin [58-60]. In addition, because patients with advanced cancer frequently present with malnutrition, there has been much interest in the relationship between hypoalbuminemia and disease status or prognosis. Many studies have suggested that preoperative hypoalbuminemia is associated with poor short-term outcomes after colorectal surgery [41, 61, 62]. Chiang et al. [61] investigated the linear relationship between preoperative albumin levels and postoperative morbidity and mortality in patients with CRC. The study reported that every 0.1-g/dL increase in albumin reduced morbidity and mortality by 7.3% and 15.6%, respectively. In another study investigating serum albumin concentrations before and after surgery for patients with CRC, it was reported that the greater the difference between albumin concentrations before and after surgery, the greater the risk of postoperative complications [63]. In addition to short-term prognosis, a number of studies have demonstrated that pretreatment albumin levels can reflect long-term oncological outcomes in patients with CRC [64-66]. In a retrospective study of 3,849 CRC patients, the 5-year OS and RFS were significantly lower in the hypoalbuminemia group with initial serum albumin of < 35 g/L than in the normal group, and postoperative morbidity was also higher in the hypoalbuminemia group [65]. Similarly, a study of CRC patients who underwent surgical treatment also found that preoperative hypoalbuminemia (< 35 g/L) was an independent poor prognostic factor of CSS [66].
C-reactive protein
CRP is a nonspecific and sensitive marker of inflammation and is produced in the liver [67]. Its synthesis is induced by proinflammatory cytokines, such as IL-6, IL-1, and TNF-α. CRP is an index that reflects the course of the inflammatory response because its value changes immediately in response to the inflammatory response. Increased CRP levels in cancer patients can predict disease severity, risk of complications, risk of tumor recurrence, and decreased survival rates [68]. According to a systematic review by Shrotriya et al. [69], increased CRP levels before surgery reflected poor prognosis in terms of short-term outcomes and oncological outcomes in patients with solid tumors in most of the studies (90%). Many studies have found that pretreatment-elevated CRP levels are associated with poor prognosis in patients with CRC patients [70-72]. Koike et al. [70] investigated the relationship between preoperative CRP level and long-term survival in patients with CRC, and the optimal cut-off value for elevated CRP was set at 0.5 mg/dL. Through multivariate analysis, the study demonstrated that CRP was an independent prognostic factor for CSS in stage I or II patients. In addition, Artaç et al. [52] analyzed whether several inflammatory markers could predict the effect of chemotherapy in metastatic CRC patients receiving chemotherapy using FOLFIRI (folinic acid, fluorouracil, and irinotecan) plus bevacizumab. In the study, CRP was measured on the day of initiation of chemotherapy, and PFS was significantly lower in the patient group above the upper limit of normal CRP level.
Glasgow Prognostic Score
GPS was introduced to enhance the effect as a prognostic predictor and is known to be a useful scoring system for predicting the prognosis of patients with CRC as well as other malignant tumors [73, 74]. GPS was based on the combination of hypoalbuminemia (< 35 g/L) and elevated CRP (> 10 mg/L); if both were abnormal, the score was 2; if one or the other was abnormal, the score was 1; if neither was abnormal, the score was 0. However, because there have been claims that hypoalbuminemia alone without elevated CRP was rare and was not associated with poor survival, GPS was modified (modified GPS [mGPS]) [74]. The mGPS was as follows: patients with CRP of ≤ 10 mg/L and albumin of ≥ 35 g/L were scored 0, those with CRP of > 10 mg/L were scored 1, and those with CRP of > 10 mg/L and albumin of < 35 g/L were scored 2. In a large retrospective study by Park et al. [75], a high score of mGPS in patients with CRC was associated with old age, emergency setting, advanced tumor stage, poor differentiation, surgical margin involvement, peritoneal involvement, and tumor perforation. The study also demonstrated that a high score of mGPS was independently associated with long-term oncologic outcomes in terms of 5-year CSS and OS. A study by Moyes et al. [76] demonstrated that preoperative mGPS was independently associated with the risk of postoperative infectious complications in patients with CRC. Besides, several studies have shown that pretreatment with high GPS or mGPS is associated with poor survival in patients with CRC [74, 75, 77]. A study by McMillan et al. [74] analyzed the relationship between mGPS and long-term survival in CRC patients who received curative resection. The study revealed that a high mGPS score is an independent prognostic factor for poor 3-year OS and CSS. In addition, a meta-analysis of 9,389 CRC patients included in 41 studies concluded that pretreatment high GPS or mGPS can reflect poor OS and CSS in CRC patients who underwent surgical resection or chemotherapy [73].
C-reactive protein/albumin ratio
Among the markers using albumin and CRP, unlike GPS and mGPS, which are classified as absolute values, CAR is a useful index indicating the ratio of albumin and CRP. Several previous studies have suggested that the CAR value can reflect the postoperative short-term outcomes in various kinds of diseases [78, 79]. There are also papers analyzing the correlation between postoperative morbidity and preoperative CAR in CRC patients. A study by Yu et al. [80] demonstrated that the preoperative CAR higher than 2.44 was an independent risk factor for anastomotic leakage. Another study by Ge et al. [81] has suggested that postoperative high CAR greater than 2.2 can be also used to predict worse postoperative outcomes in patients undergoing colorectal surgery. In addition, a study has reported that pretreatment CAR can even predict the side effects of adjuvant chemotherapy in patients with stage III CRC [82]. As well as short-term outcomes, there have also been many studies of CAR as a predictor of long-term oncological outcomes in patients with CRC [77, 83, 84]. A study by Shibutani et al. [85] demonstrated that preoperative high CAR greater than 0.0271 was independently associated with poor CSS. The study also suggested that CAR may be superior to mGPS for predicting survival. A study conducted in unresectable metastatic patients with CRC who received chemotherapy set the optimal cut-off value of pretreatment CAR to 0.183 and reported that pretreatment CAR is a useful marker for predicting OS and effectiveness of chemotherapy. The study also suggested that CAR had a better predictive value than mGPS [50]. These studies said that although mGPS is a simple and useful prognostic scoring system, it has a weak point of not reflecting the linear change. In addition, a meta-analysis with 2,492 CRC patients who received curative resection or chemotherapy in 9 retrospective studies concluded that pretreatment high CAR is a powerful prognostic indicator predicting poor OS and DFS [86-90] (Table 3).
SUMMARY AND CONCLUSION
In summary, we have reviewed that currently-available pretreatment laboratory markers could be utilized as an adjunct to predict prognosis in patients with CRC (Table 4). In literature, study subjects were colon cancer only, rectal cancer only, colon and rectal cancer together, and metastatic CRC.
In patients with colon cancer, pretreatment blood chemistry reflects short-term and long-term oncological outcomes. Hypoalbuminemia correlated with inferior OS and RFS as well as worse postoperative morbidity and mortality. In patients with rectal cancer, pretreatment CBC can predict short-term and long-term outcomes, and blood chemistry can reflect long-term oncologic outcomes. Elevated NLR and thrombocytosis correlated with higher postoperative complication rates and poor OS and DFS. In addition, high CAR was associated with poor OS and DFS.
According to studies enrolling both colon and rectal cancer patients, pretreatment CBC and chemistry tests can also reflect short- and long-term oncologic outcomes. Elevated NLR was correlated with increased length of hospital stay, poor CSS and RFS, while thrombocytosis was related to poor CSS and DFS. Hypoalbuminemia was associated with an increased hospital stay, increased postoperative 30-day mortality, and poor OS and DFS. Preoperative high mGPS predicted increased postoperative infectious complications and worse OS and DFS. Moreover, a high CAR was associated with a poor OS.
In patients with metastatic CRC, pretreatment CBC and chemistry tests can also predict long-term oncologic outcomes. Increased NLR and PLR were associated with poor OS, DFS, and PFS. High CAR and low albumin/globulin ratio on blood chemistry tests were related to poor OS and PFS.
In conclusion, a higher inflammatory status reflected by pretreatment CBC and chemistry tests can predict poor surgical and oncological outcomes in patients with either nonmetastatic or metastatic CRC. However, to be incorporated in clinical practice as a helpful adjunct, the optimal cut-off values of these laboratory markers need to be defined in future clinical studies.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF 2017R1D1A3-B03032301).