International Society of University Colon and Rectal Surgeons survey of surgeons’ preference on rectal cancer treatment
Article information
Abstract
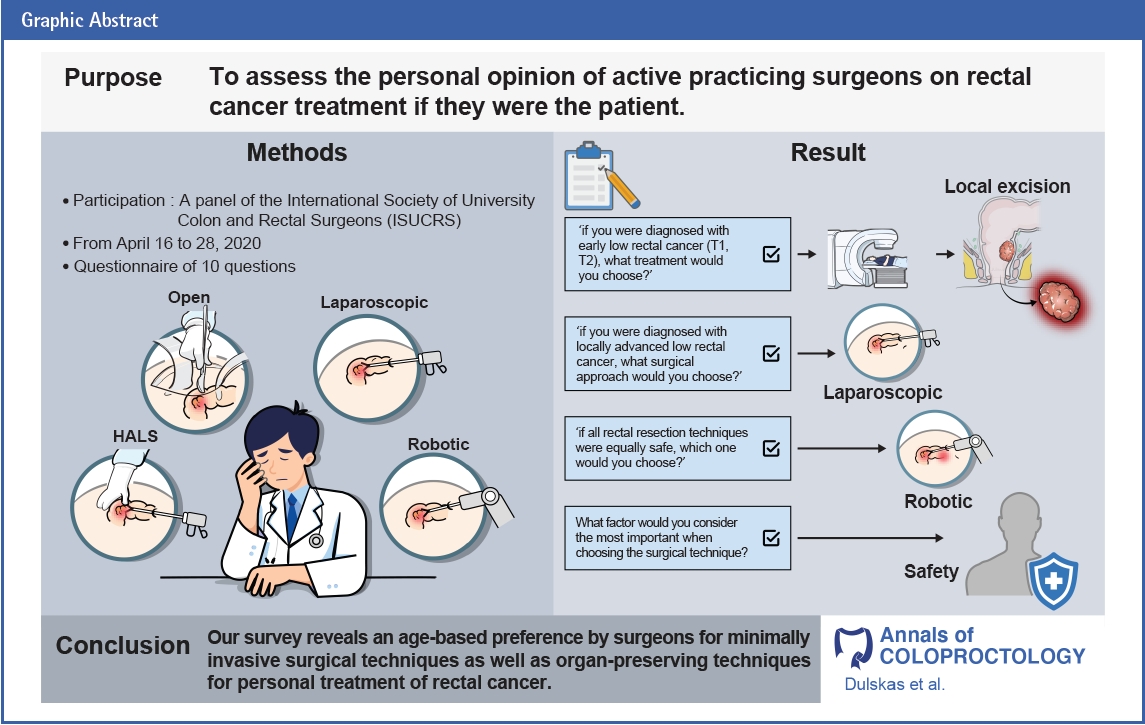
Purpose
Rectal cancer treatment has a wide range of possible approaches from radical extirpative surgery to nonoperative watchful waiting following chemoradiotherapy, with or without, additional chemotherapy. Our goal was to assess the personal opinion of active practicing surgeons on rectal cancer treatment if he/she was the patient.
Methods
A panel of the International Society of University Colon and Rectal Surgeons (ISUCRS) selected 10 questions that were included in a questionnaire that included other items including demographics. The questionnaire was distributed electronically to ISUCRS fellows and other surgeons included in our database and remained open from April 16 to 28, 2020.
Results
One hundred sixty-three specialists completed the survey. The majority of surgeons (n=65, 39.9%) chose the minimally invasive (laparoscopic) surgery for their personal treatment of rectal cancer. For low-lying rectal cancer T1 and T2, the treatment choice was standard chemoradiation+local excision (n=60, 36.8%) followed by local excision±chemoradiotherapy if needed (n=55, 33.7%). In regards to locally advanced low rectal cancer T3 or greater, the preference of the responders was for laparoscopic surgery (n=65, 39.9%). We found a statistically significant relationship between surgeons’ age and their preference for minimally invasive techniques demonstrating an age-based bias on senior surgeons’ inclination toward open approach.
Conclusion
Our survey reveals an age-based preference by surgeons for minimally invasive surgical techniques as well as organ-preserving techniques for personal treatment of treating rectal cancer. Only 1/4 of specialists do adhere to the international guidelines for treating early rectal cancer.
INTRODUCTION
Colorectal cancer remains a major cause of mortality globally [1]. The current status of treatment of patients with rectal cancer requires a multidisciplinary approach that is relatively standardized. For early rectal cancer stage T1 or T2, local excision and close surveillance or adjuvant chemoradiotherapy or radical surgery may be advised according to possible good versus poor prognostic factors [2, 3]. Whereas historically treatment for locally advanced (T3/T4 or node-positive) rectal adenocarcinoma has evolved to require fluorouracil (5-fluorouracil or capecitabine)—based neoadjuvant chemoradiotherapy followed by extirpative total mesorectal excision (TME), rectal resection with or without postoperative adjuvant chemotherapy [4–10]. As the oncological outcomes improve, whether secondary to improved chemotherapy, radiation or surgical techniques, and the rate of survival increases studies have revealed a significant degree of bowel dysfunction following rectal surgery possibly affecting as much as 90% of patients [11–13]. Moreover, the dysfunction [14] may be long term and therefore many investigators prefer total neoadjuvant therapy with watch-and-wait strategy for patients with complete clinical response [15–18].
Several surveys regarding rectal cancer treatment options have recently been conducted [19–23]. Several studies address insufficient or incomplete or biased patient-physician communication as well as the varying opinions regarding treatment between these 2 groups [19, 21, 22]. Furthermore, several surveys demonstrate the issue of lack of consensus in terms of rectal cancer treatment regarding all parties [20, 23]. All of the studies above addressed rectal cancer treatment issues based on physicians’ opinions as medical professionals for their patients but none questioned what the treatment would be if the surgeon was the patient.
With this as a foundation, the research arm of the International Society of University Colon and Rectal Surgeons (ISUCRS) conducted a global survey of surgeons who manage colorectal cancer to assess the surgeons’ preference for rectal cancer treatment with the assumption of the surgeon as patient. Our hypothesis was that surgeons being a patient would prefer minimally invasive surgery (MIS) with tendency for local excisions and watch-and-wait strategies avoiding radical surgery.
METHODS
All procedures involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This article does not contain any studies using animals. The survey filling process was totally voluntary and anonymous and it was available online. The study was exempted from the institutional ethics committee’s approval as this was totally voluntary and anonymous.
To reveal a surgeon’s preference regarding rectal cancer management, ISUCRS designed an open online questionnaire that addressed 10 questions with different clinical scenarios in addition to other demographic data (questionnaire can be found in Supplementary Material 1). A panel of members of ISUCRS developed these questions regarding rectal cancer treatment. The voluntary questionnaire was widely distributed via the ISUCRS database by emails (300 international surgeons) and was advertised on ISUCRS social media platforms including Facebook, colorectal surgeons, and LinkedIn. The anonymous questionnaire was open from April 16 to 28, 2020. There were 166 respondents. Three respondents (2 hepatobiliary surgeons and 1 medical oncologist) were excluded. The users were informed of the survey goal and the length of data storage (1 year). No personal data was collected.
We defined “abdominal surgeon” (or “gastrointestinal surgeon” as sometimes called in the United States) as a surgeon treating gastrointestinal conditions. The survey was tested for consistent reliability. It was administered to 10 random surgeons for repeating the survey 10 to 14 days later. The results were compared using Cronbach α. It is more than 0.7; the consistency is acceptable. We used CHERRIES (Checklist for Reporting Results of Internet E-Surveys). To prevent a single user from filling survey multiple times, we used IP address analysis. All users had to answer all the questions.
All statistical analyses were performed using IBM SPSS ver. 20 (IBM Corp). The chi-square test or Fisher exact test was used to compare qualitative variables, and the Student t-test or the Mann-Whitney U-test was used to analyze quantitative variables between the groups.
RESULTS
Fifteen of the survey participants (9.2%) were female, 141 (86.5%) were male, and 7 (4.3%) were not specified gender status. The surgical specialty distribution was 109 colorectal surgeons (66.9%), 37 general surgeons (22.7%), 8 abdominal surgeons (4.9%), and 9 others (5.5%; 6 surgical oncologists, 2 pediatric surgeons, and 2 gastrointestinal surgical endoscopists). The majority of responders were at age of 35 to 50 years (n=101, 62.0%). Majority of responders were from China (n=77, 47.2%), Lithuania (n=23, 14.1%), and the United States (n=23, 14.1%). Other respondents’ countries consisted of Denmark, Egypt, Ecuador, India, Russia, Germany, Greece, Italy, Korea, Japan, Poland, Romania, Turkey, the United Kingdom, Ukraine, Belgium, Brazil, Chile, Colombia, Sri Lanka, and Thailand. Twenty-six participants (16.0%) responded positively considering choice of preferring open rectal resection for their personal treatment even if minimally invasive techniques are available. Moreover, using logistic regression, we found a statistical significance between the physician’s age and open rectal resection preference (P=0.016). Furthermore, there was an agebased bias of older physicians to select open extirpative rectal resection (coefficient β=0.063887>0). The majority of the surgeons (n=135, 82.8%) were under 50 years of old.
Priority for open rectal resection differs among different specialties also (86.2% of colorectal surgeons would undergo only MIS compared to 100% of abdominal surgeons). Respondent’s specialty was found to be significant considering the preference for the open procedure (P<0.05) (Table 1). Thirty-five respondents (21.5%) were in their early years of practice (<5 years); 30 (18.4%) had 5 to 10 years; 31 (19.0%) had 10 to 15 years; 28 (17.2%), had 15 to 20 years; and 38 (23.3%) had >20 years of practice. One respondent left a blank section.
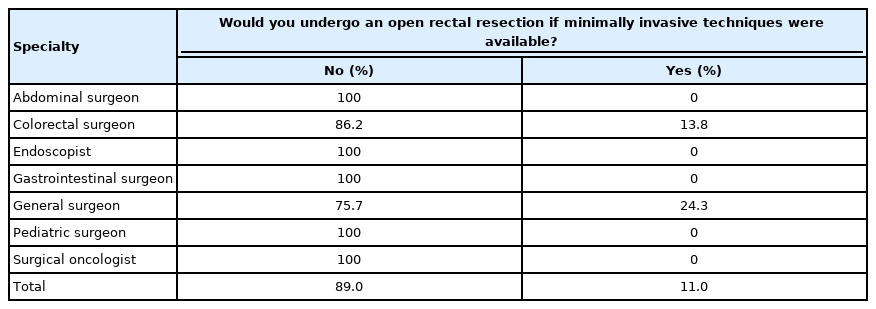
The selection of open rectal resection versus minimally invasive procedures distribution among respondent’s specialties
Considering early low rectal cancer (T1, T2) treatment, most of the respondents (36.8%) selected organ preservation with standard chemoradiation + local excision (Fig. 1). Regarding the treatment of locally advanced low rectal cancer, the first-choice treatment among the responders was laparoscopic surgery with robotic surgery following in second place (Fig. 2).
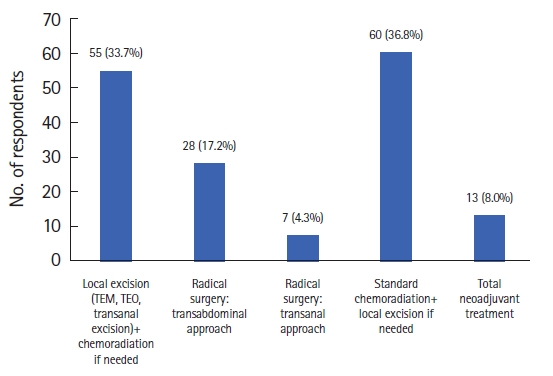
Distribution of the answers to the question, “If you were diagnosed with early low rectal cancer (T1, T2), what treatment would you choose?" TEM, transanal endoscopic microsurgery; TEO, transanal endoscopic operation.
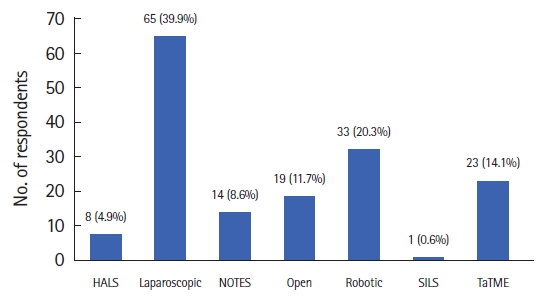
Distribution of the answers to the question, “If you were diagnosed with locally advanced low rectal cancer, what surgical approach would you choose?” HALS, hand-assisted laparoscopic surgery; NOTES, natural orifice transluminal endoscopic surgery; SILS, single incision laparoscopic surgery; TaTME, transanal total mesorectal excision.
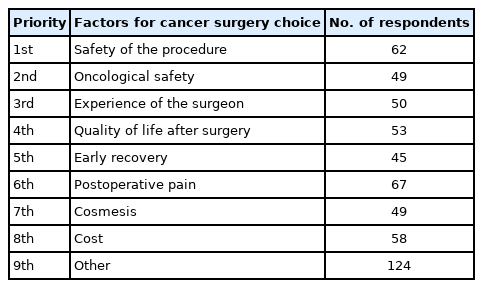
Seventy-five respondents of the participating physicians (46.0%) selected robotic surgery when all given surgical treatment techniques were told to be equally safe (Fig. 3). Robotic surgery was preferred by 75 out of 163 surgeons (46.0%) of the varying minimally invasive techniques (Fig. 4). Whereas, single incision laparoscopic surgery (SILS) was ranked second and conventional laparoscopic surgery (CLS) third among the respondents. Respondents were also asked to select priorities for surgical rectal procedures. The highest priority consideration of our respondents was safety of the procedure and then the oncological safety (Table 2). Further priorities were arranged as follows: experience of the surgeon, quality of life after surgery, early recovery, postoperative pain, cosmesis, and cost.

Distribution of the answers to the question, “If all rectal resection techniques (open, hand-assisted laparoscopic surgery [HALS], laparoscopic, robotic, single incision laparoscopic surgery [SILS], and natural orifice transluminal endoscopic surgery [NOTES]) were equally safe, which one would you choose?"
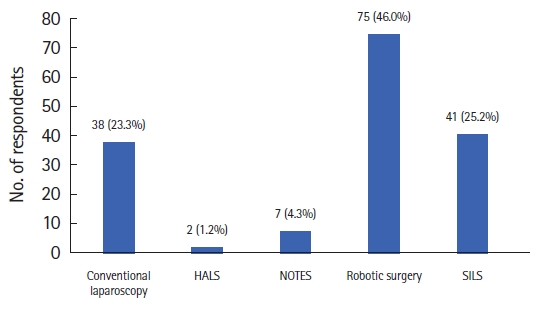
Distribution of the answers to the question, “If only the minimally invasive techniques were offered to you (hand-assisted laparoscopic surgery [HALS], laparoscopic, robotic, single incision laparoscopic surgery [SILS], and natural orifice transluminal endoscopic surgery [NOTES]), which one would you choose?"
We also found that the questionnaire had a good consistency (Cronbach α, 0.95).
DISCUSSION
In this prospective collected study, we showed that surgeons if they were presented with rectal cancer, chose minimally invasive and organ-preserving techniques more often if they were making decisions for themselves as patients. In regards to T1 or T2 rectal cancer, surgeons chose local excision with standard chemotherapy preferentially. Laparoscopy was the first-choice approach for locally advanced cancer. The best minimally invasive approach was selected to be robotic.
We found that older surgeons are prone to doing open despite if minimally invasive techniques are available. This is consistent with other studies; open radical surgery trending toward older surgeons and advanced laparoscopic procedures are performed by younger specialists [24]. However, this underutilization of MIS may be due to the lack of training possibilities globally [25]. Along with expanding indications for MIS techniques [26, 27] and growing international experience with minimally invasive approaches [28], these recommendations and experiences are being shared for the MIS training [29, 30].
While radical surgery is a treatment of choice according to the European Society for Medical Oncology (ESMO) and National Comprehensive Cancer Network (NCCN) rectal cancer treatment guidelines [31, 32], only 21% of our respondents chose this option. Obviously, this is influenced by not subdividing the early rectal cancer to very early (T1 without poor prognostic factors, where local excision is sufficient). Selection by the respondents of organ-preserving and minimally invasive conservative treatment was possibly related to the fact that radical surgery is associated with a high percentage of postsurgical complications (3%–30% [33]) and organ dysfunction (defecation disorder up to 75%–90%, sexual dysfunction up to 50%, and urination dysfunction up to 30% [13, 34–40]). Moreover, bowel dysfunction is a long-lasting issue [14]. Considering this, organ-preserving treatment techniques are increasing in popularity, such as local excision, chemoradiation with local excision, or only total neoadjuvant treatment as preferred treatments [41–43]. In addition, our responders chose laparoscopy and robotics as the first choice for the treatment of locally advanced rectal cancer. The probable justification for the choices is the wide availability of the laparoscopic approach, in contradistinction to robotic surgery. This may reflect the issue that robotic surgery is more expensive, at present, not available internationally in underdeveloped countries and that the operative time is longer [44]. Moreover, the cost and lack of robotic wider spread can be associated with the absence of strong, evidence-based advantages for the patient over the conventional laparoscopy. In the systematic review and meta-analysis by Prete et al. [45], authors showed that the robotic approach for rectal cancer is indistinguishable from conventional laparoscopic treatment considering perioperative oncological procedure oncologic results, although the operating time was significantly longer. However, the learning curve for younger surgeons is shorter and this may consequently make robotic surgery preferred by this group [46]. The experience may be the result of a lower conversion rate to open surgery compared with conventional laparoscopy [45]. On the contrary, the better quality of robotic TME, better urinary function outcomes, lower blood loss (15.4±26.4 mL vs. 39.1±85.1 mL) and conversion rate (0% vs. 3.3%), and shorter hospital stay (7.3±2.3 days vs. 9.3±6.7 days) in comparison with laparoscopic TME were being reported [44]. After all, bowel function and quality-related advantages of robotic TME may in the long-term cause a greater oncological safety. Novel transanal TME (TaTME) sometimes may be attributed to natural orifice transluminal endoscopic surgery (NOTES) [47]. In terms of pathological outcomes, TaTME may be superior to laparoscopic TME, with lower circumferential resection margin and distal resection margin positivity rates [48]. The transanal approach showed better results over laparoscopic techniques in regards to readmission rate (9% after TaTME vs. 18% after laparoscopic TME), major and overall morbidity (8.7% vs. 14% for major morbidity and 34% vs. 41% for overall morbidity), length of hospital stay (95% confidence interval, 3.68–0.66), and anastomotic leak rate (6.4% vs. 11.6%) [44, 49]. It is important to state that most studies are based on nonrandomized trials, and data regarding oncological TaTME safety from multicenter studies is inconclusive, at present [44, 49, 50]. Therefore, excluding unreliable NOTES safety criteria and given better cosmetic and perioperative outcomes makes this procedure one of the top choices. Surgical morbidity, oncological suitability, cost, intraoperative bleeding, rate of conversion to open surgery, anastomotic leakage rate, readmission, local recurrence, and distal metastases rates are similar in SILS and CLS [51, 52]. Nevertheless, SILS seems to offer some advantages in relation to a smaller incision, such as shorter length of hospital stay, better cosmetic results, faster return of bowel function, reduced postoperative pain, and overall complication rate [46, 47]. These considerations may reflect the preference in the survey for CLS.
Our respondents answered that surgical and overall safety was the primary goal of the surgery. Other recent studies showed similar tendency; surgeons base treatment decisions on existing information about specific surgical method safety, clinical experience, and patient medical condition preintervention and postintervention [53, 54].
The strength of our study is the novel and original approach to the perceptions by the surgeons of the rectal cancer treatment. Moreover, we have included a high number of homogenous specialists from different countries. However, our study has some limitations. First, the low response rate (54.3%) might not reflect the true experience globally. Relatively low survey response rates are a major and growing problem worldwide. Obviously, they can bias survey results by introducing nonresponse error. Low response rates can be the result of a number of factors including survey mode; nonworking email addresses as all the respondents were reached through emails, the online-based questions, which sometimes might be hard filling for older generation surgeons. Another explanation might be inactivity in these kinds of trials. Second, we could not assess the validity of the survey. Theoretically, the survey results might be different if the respondents really had cancer. However, this is only hypothetical predictions and this was not the goal of our study.
To conclude, our survey revealed an age-based preference by surgeons for minimally invasive surgical techniques as well as organ-preserving techniques for personal treatment of treating rectal cancer. Only one-fourth of specialists do adhere to the international guidelines for treating early rectal cancer.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Acknowledgments
The authors wish to thank each and every one who has taken the time and effort to complete the questionnaire. Their contribution is requisite for the study and is therefore highly appreciated.
Author contributions
Conceptualization: AD; Data curation: all authors; Formal analysis: DG, LZ; Visualization: AD, LZ, RF; Writing–original draft: AD, DG, JWNM, PFC; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Additional information
This study was presented as a poster at the European Society of Coloproctology (ESCP) conference on September 21–24, 2021, in Barcelona, Spain.
Supplementary materials
Supplementary Material 1.
Survey questionnaire.
Supplementary materials are available from https://doi.org/10.3393/ac.2022.00255.0036.