Validation of the Vietnamese version of the low anterior resection syndrome score questionnaire
Article information
Abstract
Purpose
The aim of this study was to validate the low anterior resection syndrome (LARS) score questionnaire in the Vietnamese language among Vietnamese patients who underwent sphincter-preserving surgery for rectal cancer.
Methods
The LARS score questionnaire was translated from English into Vietnamese and then back-translated as recommended internationally. From January 2018 to December 2020, 93 patients who underwent sphincter-preserving surgery completed the Vietnamese version of the LARS score questionnaire together with an anchored question assessing the influence of bowel function on quality of life (QoL). To validate test-retest reliability, patients were requested to answer the LARS score questionnaire twice.
Results
Ninety-three patients completed the LARS score questionnaire, of whom 89 responded twice. The patients who responded twice were included in the analysis of test-retest reliability. Fifty-eight patients had a “major” LARS score. The LARS score was able to discriminate between patients who were obese and those who were not (P<0.001) and between the low anterior resection and anterior resection procedures (P<0.001). Age and sex were not associated with higher LARS scores (P=0.975). There was a perfect fit between the QoL category question and the LARS score in 56.2% of cases, and a moderate fit was found in 42.7% of cases, showing reasonable convergent validity. The test-retest reliability of 89 patients showed a high intraclass correlation coefficient.
Conclusion
The Vietnamese version of the LARS score questionnaire is a valid tool for measuring LARS.
INTRODUCTION
The outcomes of rectal cancer worldwide have dramatically improved for decades [1]. Implementing an early diagnosis policy, applying chemoradiation, and performing sphincter-preserving surgery more frequently have yielded excellent outcomes for rectal cancer patients. Together with a high survival rate, survivors following radical surgery for rectal cancer suffer from low anterior resection syndrome (LARS) and symptoms including frequent bowel movements, gas and fecal incontinence, fragmentation, and urgency [2, 3]. Of various oncologic outcomes, quality of life (QoL) is an important measure for rectal cancer treatment [4, 5]. Among several tools and questionnaires published to evaluate LARS, the LARS score by Emmertsen et al. [6] has shown feasibility and validity for recording LARS. The LARS score precisely reflects the severity of bowel disorders after sphincter-preserving surgery by scoring the symptoms of LARS [2]. After the original LARS score was published in Danish, this questionnaire was validated in many languages, including English, Slovene, Dutch, Greek, Lithuania, Korean, Japanese, Chinese, and Persian [7-14]. There has been, however, no validated Vietnamese version of the LARS score questionnaire. The aim of this study was to develop a Vietnamese version of the LARS score questionnaire and evaluate its convergent validity, discriminative validity, and reliability.
METHODS
Ethics statement
This study was approved by the Institutional Review Board of Nhan dan Gia Dinh Hospital (No. was 66/NDGĐ–HĐĐĐĐ). All recruited patients signed a written informed consent form for participation in the study.
Participants
The electronic records of a public tertiary hospital were searched for patients with rectal cancer who were treated with sphincterpreserving surgery between January 2018 and December 2020. All patients, 18 years and older, operated for rectal cancer within 15 cm from the anal verge by sphincter-preserving procedures (anterior resection [AR], low anterior resection [LAR], intersphincteric resection, and LAR with the transanal approach) were included. The exclusion criteria were the formation of a stoma, known disseminated or recurrent disease, inability to read and write in Vietnamese, or any psychiatric conditions that might affect the questionnaire assessment. In January and March 2021, questionnaires regarding bowel function were sent to all 172 eligible patients identified in our database, who had undergone all kinds of sphincter-preserving surgery for rectal cancer. Demographic and clinical data were obtained from patients’ medical files.
Methods
Description of the LARS score
Bowel function was assessed with the LARS score. A 5-question questionnaire evaluated functional symptoms. The questions and scoring system of the LARS score questionnaire in the Vietnamese language are shown in Appendix 1. Scores were assigned to possible responses in order to calculate the LARS score, which was allocated into “no LARS” (score of 0–20 points), “minor LARS” (21–29 points), and “major LARS” (30–42 points) [11]. All questions were required for inclusion in our analysis.
Translation into Vietnamese
The translation procedure followed international guidelines, and included independent forward- and back-translations, as well as adaptive testing of the final Vietnamese version [15-17]. Two independent professional translators, both native Vietnamese speakers, carried out the forward translation (English to Vietnamese). The translators discussed any differences between the 2 versions to reach a final version. This was then back-translated (Vietnamese to English) by a third independent native English translator who had not seen the original English version. The final Vietnamese version was only approved by the study team when the differences between the back-translated English version and the original English version were resolved.
Psychometric validation
1) Test-retest reliability
The consistency and the ability to achieve consistent results between the first and second responses were classified as perfect, moderate, and no fit. Perfect fit was defined as the same responses for both the first and second interview. A difference in 1 or 2 categories was classified as moderate or no fit, respectively.
2) Validity
Convergent validity refers to agreement between measures that are supposed to be related, and it is evaluated using different methods. The convergent validity of the Vietnamese LARS score was verified by adding an anchored question (“Overall, how much does your bowel function affect your quality of life?”) to observe the association between LARS score and QoL. This question was sent with the LARS score questionnaire. The answers for the anchored QoL question were “no impact,” “a little,” “some,” and “a lot.”
Discriminative validity measures the ability to discriminate between groups with known differences. Discriminative validity was evaluated by assessment of the following groups in the LARS numerical score: presence or absence of obesity, type of surgery (AR vs. LAR), sex (male or female), and being older or younger than the mean age of the participants.
RESULTS
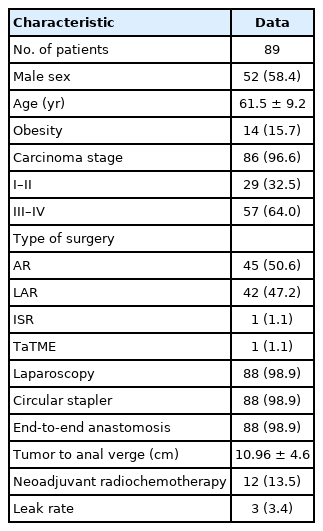
Study participants’ characteristics
Out of 172 patients eligible for the study, 93 (54.1%) responded. After 14 days, the questionnaire was sent again to all responding participants. Out of those, 89 participants (95.7%) responded a second time. Overall, 89 participants were included in the test-retest analysis. There were 37 (41.6%), 31 (34.8%), and 21 participants (23.6%) with no LARS, minor LARS, and major LARS, respectively (Table 1).
Psychometric validation
Test-retest reliability
As shown in Table 2, each of the 5 categories presented a high proportion of perfect fit (85.4%–96.6%). The intraclass correlation coefficients (ICCs) ranged from 0.874 to 0.934, showing high agreement. Fig. 1 shows a Bland-Altman plot of the extent of agreement between the first and second LARS scores. The percentages with 95% confidence intervals (CIs) of perfect, moderate, and no fit between the first and second responses to each of the LARS score questions are shown in Table 2.
Convergent validity, sensitivity, and specificity
According to the QoL categorization, perfect, moderate, and no fit were found in 56.2%, 42.7%, and 1.1% of responses, respectively (Table 3). The agreement between the 2 groups was the highest (25.8%) for minor LARS and minor impact of bowel function on QoL. The percentages of perfect, moderate, and no fit between the LARS and QoL groups with 95% CIs are shown in Table 4. No fit was found in 1.1% of the data.
Fig. 2 presents a box plot showing that the QoL groups had statistically significant differences in the LARS numerical score (P< 0.001). The post hoc difference was found between all levels of impact of bowel function in the QoL group (P< 0.001).

Differences among groups of numerical low anterior resection syndrome (LARS) scores and groups of quality of life.
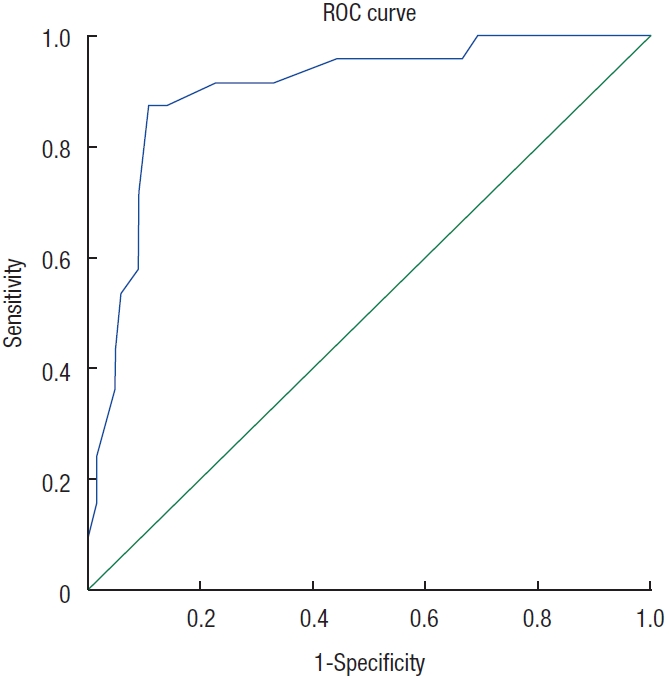
The sensitivity and specificity of the LARS score in differentiating patients with a moderate or severe impact on QoL from those with no or a minor impact on QoL were 87.5% and 89.2%, respectively. This shows that the LARS score could predict the influence of symptoms related to LARS on QoL. The receiver operating characteristic curve of the LARS scores predicting patients with a moderate or severe influence on QoL revealed an area under the curve of 0.902 (Fig. 3).
Discriminative validity
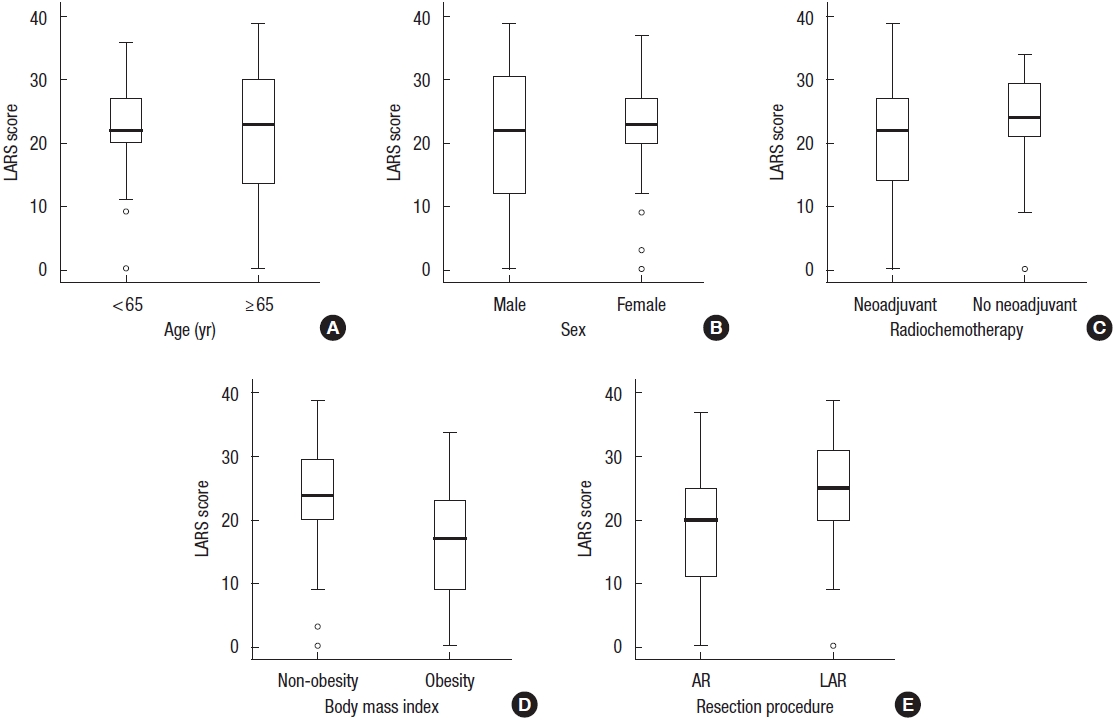
Obesity and the AR procedure were associated with significantly lower LARS scores. The LARS score was not significantly different among groups according to sex, age, or adjuvant radiochemotherapy (Fig. 4).
DISCUSSION
This study developed a cross-culturally appropriate Vietnamese translation of the LARS score and revealed the validity and reliability of the questionnaire. The LARS score questionnaire was shown to be an easily understood, simple, and applicable tool for LARS assessment. The results of this study were close to those of published validation studies. As such, it was confirmed that the Vietnamese LARS score is a cross-culturally equivalent tool to the original version.
The test-retest reliability was excellent, with ICCs ranging from 0.874 to 0.934, indicating minimal measurement error and high internal consistency of the translated version. These results are comparable to the Danish, Lithuanian, Dutch, English, Korean, Japanese, and Chinese results [8, 9, 11, 18].
A meticulous translation and cross-cultural adaptation process was followed to provide an equivalent Vietnamese version of the LARS score. An anchored QoL category question was added to validate the Vietnamese LARS score. The proportion of our patients with a perfect fit between the LARS score and QoL was 56.2%, which is comparable to the results reported by other authors, ranging from 41% to 63% [12-14]. The percentage of respondents with “no fit” between the LARS score and QoL was lower in our study than in other studies, in which it was up to 8% [12-14].
The sensitivity and specificity of the previously validated version have been reported to be up to 88.3% and 88.8%, respectively [19]. The Vietnamese LARS score showed reasonable sensitivity (87.5%) and specificity (89.3%) compared to previous publications.
Finally, the LARS score was higher in patients who were not obese and had undergone LAR, which is in agreement with the results of other studies [9, 11, 14]. Since it has been shown that sphincter-preserving surgery, such as AR and LAR, has negative effects on anorectal function, resulting in LARS [19], our study confirmed that patients who underwent LAR would develop LARS more frequently than those who underwent AR [20].
The main limitation of our study is that it is a single-center study at a tertiary hospital. To draw a better picture of functional complaints in Vietnamese patients, a multi-center study is required. Another weak point of this study is that the LARS score was not able to discriminate patients who had undergone radiotherapy, even though radiotherapy has been proven to have a strong relationship to LARS [20].
Local control and long-term survival are the primary therapeutic goals of rectal cancer surgery. However, due to concerns about QoL after surgery, LARS must be a concern in the management of rectal cancer prior to surgical treatment.
In conclusion, a valid Vietnamese version of the LARS score questionnaire is now available and can be applied with confidence to recognize and follow up with patients who have anorectal disorders after rectal surgery. The psychometric properties indicate that the Vietnamese version of the LARS score is valid, consistent, and reliable. This also strengthens the evidence that the LARS score is a strong and valid tool for the assessment of QoL in patients who have undergone sphincter-preserving operations for rectal cancer.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.
AUTHOR CONTRIBUTIONS
Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Visualization: TAMP. Data curation, Investigation: VQP. Writing–original draft: TAMP. Writing–review & editing: TAMP, VQP.
All authors have read and approved the final manuscript.
Acknowledgements
The authors would like to thank Dr. Tran Trong Tan, Dr. Le Kim Long, and Dr. Do Thi Thu Phuong for their support.