Endoscopic transstomal stent insertion: a novel approach for a stenosed stoma in a challenging patient
Article information
Abstract
Transstomal stent deployment to maintain the patency of stoma in a challenging patient who developed stoma stenosis, is a minimally invasive, novel technique. This is a new and alternative approach in management of stoma stenosis in a difficult case using a biodegradable stent for end colostomy.
INTRODUCTION
Colostomy stenosis is a potential late complication, with a reported incidence between 2% and 14% [1–3]. The risk factors for this complication include compromised blood supply, obesity, infection, and ostomy retraction [2]. Its management includes definitive treatment by surgery with refashioning of the stoma. In this paper, we discuss a novel approach to the management of stoma stenosis. To date, there are insufficient data in the literature with regard to similar management strategies. We suggest that this treatment option for stoma stenosis could be a suitable alternative in challenging patients and those who are unfit for surgical stoma revision or refashioning. Of particular note, this technique can be implemented with a minimally invasive intervention and no need for general anesthetic [4].
TECHNIQUE
Ethics statement
After a thorough discussion with the patient, we obtained verbal and written consent to perform our proposed new approach. The patient also provided written informed consent for publication of the research details and clinical images. This procedure did not need an Institutional Review Board approval, as the stent we used for this procedure is already available for varied gastrointestinal tract strictures management and we did not use any experimental material which may require ethical approval.
Case briefing
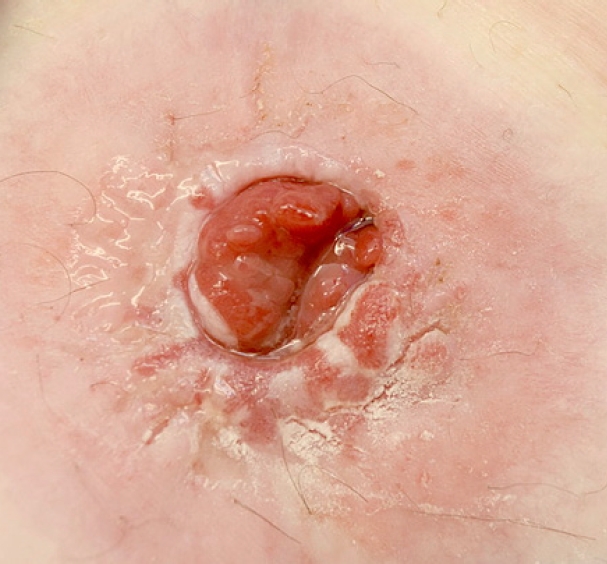
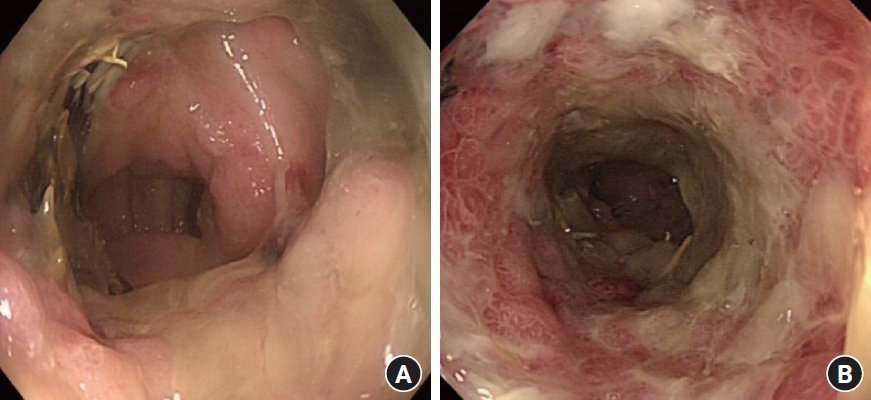
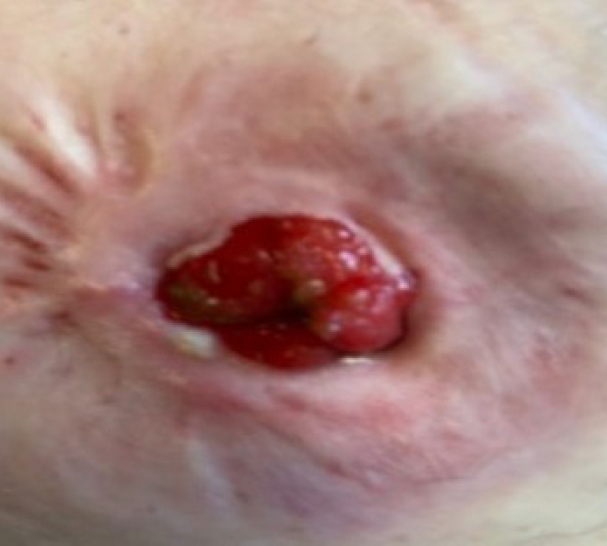
A 61-year-old man with a known history of hypertension and diabetes mellitus, who was an ex-smoker with a 30-year smoking history, and had morbid obesity with a body mass index of 50 kg/m2, previously underwent a difficult laparoscopic abdominoperineal resection of low rectal cancer following neoadjuvant chemoradiotherapy. The abdominal wall thickness through which a bulky colon with its fatty mesentery was pulled was about 15 cm. The patient developed stoma necrosis in the immediate postoperative period and subsequently early stoma stenosis (Figs. 1, 2). Stoma revision would have been a challenging task, both due to the patient’s body habitus and comorbidities and the high risk of recurrence of a similar problem [5]. The initial assessment showed a viable colonic mucosa sitting 3 cm away from the skin level, and a conservative approach was started with the help and support of stoma care nurse specialist to teach the patient to perform regular dilatation. However, despite this initial treatment plan, 4 weeks later there was ongoing stenosis, and the patient complained of abdominal pain and distention with a reduction of stoma output (once every 3–4 days). Early signs of obstruction were noted; accordingly, the patient’s quality of life was affected, and he required regular visits to the stoma care clinic and analgesics. On further assessment, stoma narrowing could not allow an examining little finger to pass, and an endoscope could not be passed to assess the status of the stoma. The decision was made to widen the stoma after obtaining verbal and written consent from the patient, after which we performed an endoscopic assessment and our new treatment approach described below.
Surgical technique
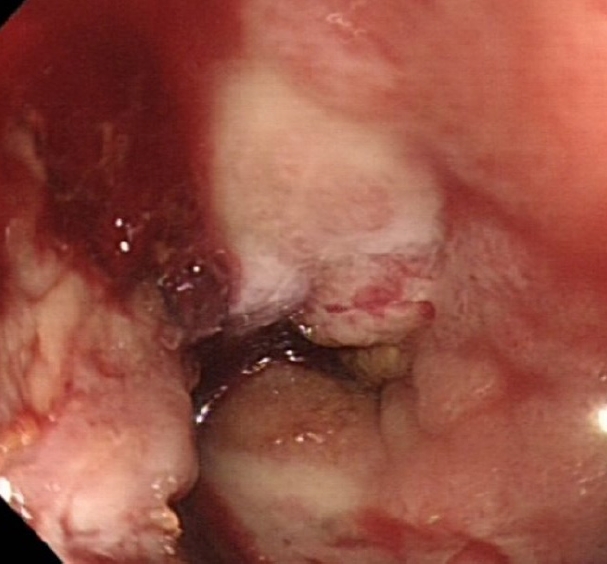
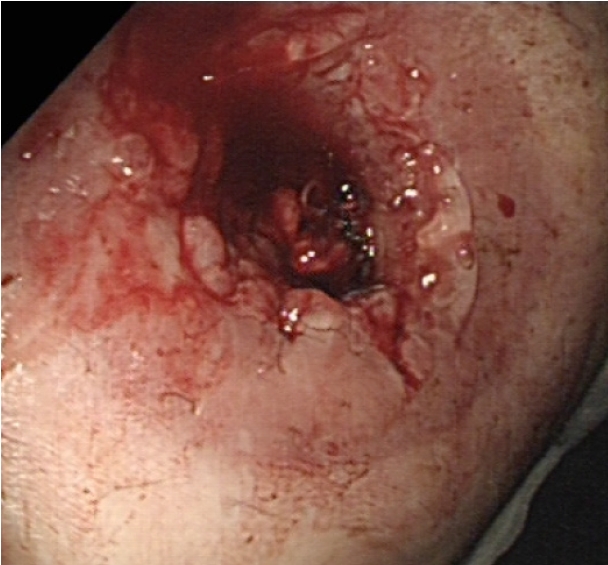
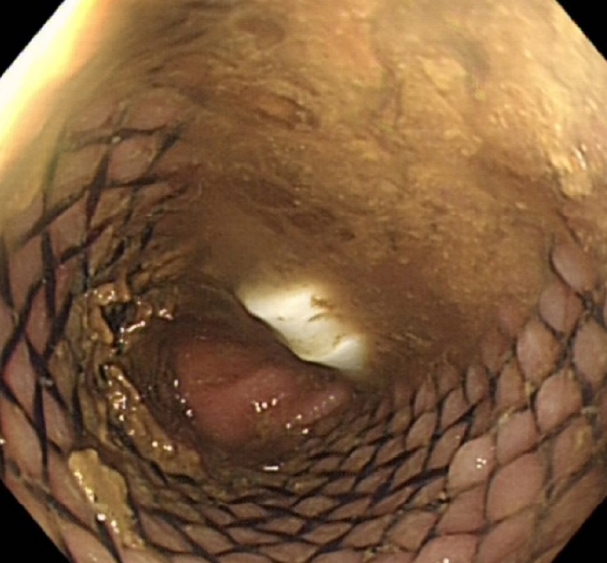
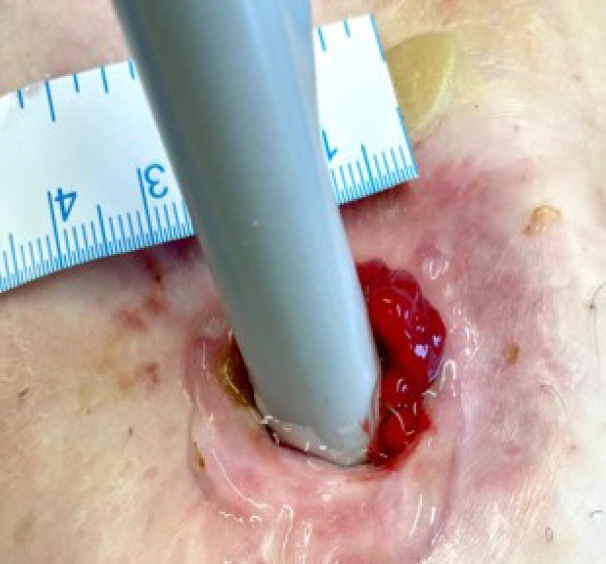
Under aseptic conditions, a povidone-iodine solution was used to clean the skin around the stoma, and local anesthesia (20 mL of 0.5% bupivacaine) was instilled as a field block to the skin around the stoma. Radial incisions of the skin and subcutaneous tissue of 1-cm length were performed at the 12-, 2-, 4-, 6-, 8-, and 10-o’clock positions (Fig. 3). This allowed us to relieve the stenosis, and a stoma opening of 3 cm was achieved. This was followed by endoscopy via the stoma, which confirmed a healthy stoma about 3 cm from the skin level. A biodegradable stent (ELLA BD Stent, UK Medical) that was 6 cm long and 3 cm wide at the upper end and with a body of 2.5 cm, was inserted under direct vision and endoscopic guidance to confirm good bridging between the healthy colon (Fig. 4) and skin edge. We ensured that the stiffer end of the stent sat against the edge of the skin and was sutured to it with 3/0 polydioxanone interrupted sutures to keep the stent at this level (Fig. 5). The patient was initially followed weekly at the stoma care clinic, and at the 3rd week, granulation tissue was seen lining the stent (Fig. 6). At the 3rd month postprocedure, the stent had nearly completely dissolved; the stoma opening measured 2.5 cm, and it remained this size for 9 months following this procedure (Fig. 7).

The stent in situ after the radial skin-releasing incisions. (A) Immediate appearance. (B) One week later.
Results
Initially, the patient experienced pain and discomfort at the stoma site for the first 4 days due to the skin incisions, which was controlled with regular paracetamol (1 g, 4 times/day). He had an immediate bowel movement and release of flatulence and continued to have regular bowel movements on a daily basis. He had some difficulty cleaning the stoma for the first 4 weeks following the procedure due to the presence of sutures holding the stent, but once the sutures were removed, he did not have any stoma care issues. Through regular outpatient follow-up with the main surgeon and stoma nurse, the patient has successfully maintained an open stoma without recurrence of stenosis, thereby avoiding the potential to undergo major surgery. The use of dilators after the stent insertion was mainly a precaution, rather than a necessity, and was done easily by the patient without impacting quality of life and inducing discomfort; it was carried out every time the patient changed the stoma bag (2 times/day). The stent dissolved in 3 months’ time (Fig. 7). The patient was compliant with our clinical advice regarding stoma care. On long-term follow-up 30 months postprocedure, minimal narrowing reappeared, but was easily managed by the patient with once-daily self-dilation. The latest assessment at the stoma care clinic demonstrated a stoma diameter of 2.5 cm with an opening of at least 2 cm (dilator size, 18 mm), and the patient had no bowel symptoms (Figs. 8, 9).
DISCUSSION
Stoma stenosis is a major complication of bowel ostomy with a relatively high incidence rate (up to 14%) [6, 7]. It usually requires definitive treatment (most commonly, surgery or repeated mechanical dilatation), which carries a high risk of recurrence, patient discomfort, and other potential complications [6]. The surgical option is not always feasible and can lead to more complications in difficult or high-risk patients. We have introduced this novel approach, using biodegradable stenting, that provides a promising additional tool for future management in high-risk patients who develop stoma stenosis. This technique can be used as either a permanent solution or as a maneuver to allow more time for patient optimization before proceeding to a planned revision or closure of the stoma in other cases, such as Hartmann procedure.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: KS;. Data curation: AG, AMM; Formal analysis: AG, KS; Investigation: KS; Methodology: AG, KS; Project administration: KS; Resources: AMM, KS; Software: AMM, FA; Supervision: KS; Validation: KS; Visualization: all authors; Writing–original draft: all authors; Writing–review & editing: all authors.
All authors read and approved the final manuscript.