Total neoadjuvant therapy in rectal cancer: a network meta-analysis of randomized trials
Article information
Abstract
Purpose
To assess the efficacy of total neoadjuvant therapy (TNT) for rectal carcinoma in comparison with conventional chemoradiotherapy (CRT).
Methods
A systematic review was performed according to the PRISMA guidelines. A Bayesian network meta-analysis was done using NetMetaXL and WinBUGS. This study was registered in PROSPERO on March 3, 2022 (No. CRD-42022307867).
Results
Outcomes of 2,719 patients from 10 randomized trials between 2010 and 2022 were selected. Of these 1,191 (44%) had conventional long-course CRT (50–54 Gy) and capecitabine, 506 (18%) had induction chemotherapy followed by CRT (50–54 Gy) and capecitabine (iTNT), 230 (9%) had long-course CRT (50–54 Gy) followed by consolidation chemotherapy (cTNT), and 792 (29%) undergone modified short-course radiotherapy (25 Gy) with subsequent chemotherapy (mTNT). Total pathologic complete response (pCR) was 20% in the iTNT group, 21% in the mTNT group, 22% in the cTNT group, and 12% in the CRT group. Statistically significant difference in pCR rates was detected when comparing iTNT with CRT (odds ratio [OR], 1.76; 95% credible interval [CrI], 1.06–2.8), mTNT with CRT (OR, 1.90; 95% CrI, 1.25–2.74), and cTNT with CRT groups (OR, 2.54; 95% CrI, 1.26–5.08). No differences were found in R0 resection rates. No significant difference was found in long-term outcomes.
Conclusion
The early administration of systemic chemotherapy in the TNT regimen has improved short-term outcomes, though long-term results are underreported. Randomized trials with survival as the endpoint are necessary to evaluate the possible advantages of TNT modes.
INTRODUCTION
Neoadjuvant chemoradiotherapy (CRT) followed by total mesorectum excision (TME) is the standard treatment in most patients with cancer of the mid and low rectum. Implementing this treatment approach reduced the rate of local recurrences from 25% to 5%–10% [1, 2]. Currently, distant metastases of rectal carcinoma are the main cause of cancer-specific death in these patients. Two meta-analyses published by Zorcolo et al. [3] and Martin et al. [4] in 2012 showed that overall survival is affected by the pathologic complete response (pCR) of the tumor after neoadjuvant CRT. A standard combination of 40 to 50 Gy and 5-fluoruracil in the preoperative setting allows a pCR rate of 10%–15% to be achieved [5].
Total neoadjuvant treatment (TNT) increases the rate of pCR. According to published studies, the rate of pCR after TNT varies from 10% to 33% [6–9]. The efficacy and tolerability of the treatment directly depend on the chosen TNT regimen, the number of chemotherapy courses, and the mode of radiotherapy (RT). However, the lack of a uniform approach to TNT complicates assessments of the efficacy and safety of this treatment. In this context, a meta-analysis of outcomes of randomized trials comparing current TNT regimens with traditional neoadjuvant CRT in patients with rectal cancer is relevant.
METHODS
Search strategy and inclusion criteria
We performed a systematic review and meta-analysis in accordance with the recommendations of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [10]. The study was registered in PROSPERO (International Prospective Register of Systematic Reviews) in 2022 (No. CRD42022307867), and the detailed prespecified protocol is available upon request.
The MEDLINE (via PubMed), Cochrane Library, and Google Scholar electronic databases were searched for original articles to analyze. The search was performed using the following search terms: (“rectal neoplasms” [MeSH Terms] OR (“rectal” [All Fields] AND “neoplasms” [All Fields]) OR “rectal neoplasms” [All Fields] OR (“rectal” [All Fields] AND “cancer” [All Fields]) OR “rectal cancer” [All Fields]) AND ((“total” [All Fields] OR “totalled” [All Fields] OR “totalling” [All Fields] OR “totaled” [All Fields] OR “totaling” [All Fields] OR “totals” [All Fields]) AND (“neoadjuvant therapy” [MeSH Terms] OR (“neoadjuvant” [All Fields] AND “therapy” [All Fields]) OR “neoadjuvant therapy” [All Fields])). In total, 2,728 English-language publications were found for the period from 1993 to 2022. We also identified 1 unpublished trial from N.N. Blokhin National Medical Research Center of Oncology (Moscow, Russia), which was also included in the network meta-analysis. After the initial analysis, duplicates, review articles, and noncomparative studies were excluded. Publications were included in the meta-analysis in accordance with the following criteria: (1) randomized trials; (2) patients with mid and low rectal adenocarcinoma without distant metastases, who underwent neoadjuvant treatment and TME; and (3) neoadjuvant treatment carried out according to 1 of the following schemes: long-course CRT (50–54 Gy) and capecitabine; induction chemotherapy followed by CRT (50–54 Gy) and capecitabine (iTNT); long-course CRT (50–54 Gy) followed by consolidation chemotherapy (cTNT); and modified short-course RT (25 Gy) followed by consolidation chemotherapy (mTNT). The studies were not limited to the delivery of adjuvant chemotherapy, regardless of the chosen neoadjuvant regimen (Fig. 1).
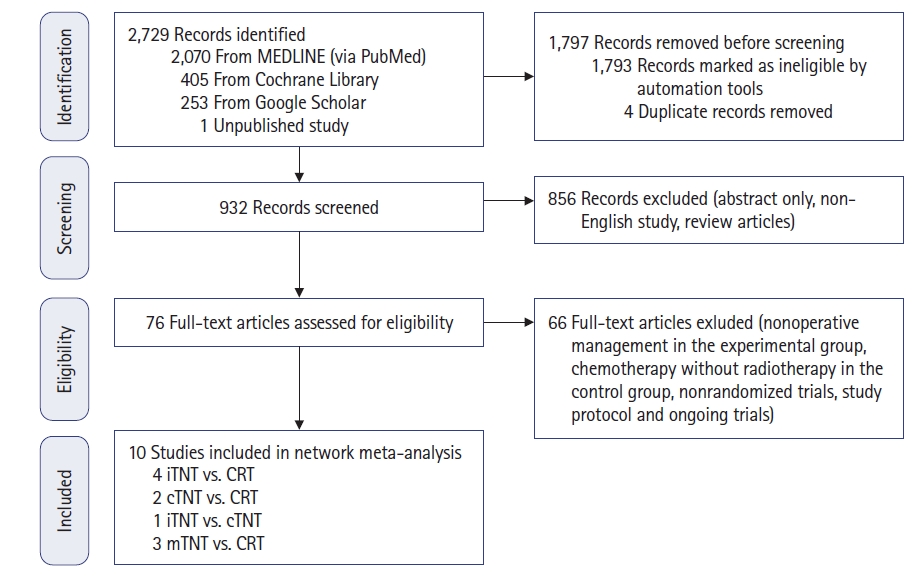
Stepwise procedures for database search and the selection of eligible studies. CRT, chemoradiotherapy; TNT, total neoadjuvant treatment; cTNT, long-course CRT (50–54 Gy) followed by consolidation chemotherapy; iTNT, induction chemotherapy followed by CRT (50–54 Gy) and capecitabine ; mTNT, short-course radiotherapy (25 Gy) followed by consolidation chemotherapy.
Assessment of methodological quality
The methodological quality of the studies included in the meta-analysis was evaluated using the Risk of Systematic Deviation Table: Risk of Bias (RoB). RoB was evaluated in accordance with the Cochrane manual [11]. The Review Manager ver. 5.3 (Cochrane) was used to create the RoB table. Two of the authors (SS and A Ponomarenko) independently evaluated the methodological quality of all studies, and any disagreements between the 2 evaluators were resolved by discussion or by consultation with a third author (ER).
Data extraction
The following data were extracted from the studies: author, year of publication, study design, number of patients in groups, characteristic of the groups, the TNT scheme, the pCR rate, the rate of R0 resections, overall survival (OS), the local recurrence rate (LRR), and the metastasis rate (MR).
Statistics
The network meta-analysis was carried out using the Bayesian analysis Monte Carlo algorithms in WinBUGS ver. 1.6.1 (MRC Biostatistics Unit) and the Microsoft Excel program (NetMetaXL, Microsoft Corp) [12]. The deviance information criterion was estimated when analyzing random models [13]. An “informative prior” parameter was chosen when calculating the statistical model with the aim of obtaining less heterogeneity [14]. The number of iterations was set to 40,000, with 20,000 iterations as burn-in. The dichotomous data are described in the form of odds ratios (ORs) and risk ratios. The effect sizes for the Bayesian network meta-analysis were described with 95% credible intervals (CrIs). Statistical significance was defined as a 95% CrI not including 1. Rankograms and surface under the cumulative ranking curve (SUCRA) were used to examine ranking probabilities. Inconsistencies in the analysis were identified via a visual examination of the posterior mean deviance of the individual data points in the inconsistency model against their posterior mean deviance in the consistency model. Confidence in the network meta-analysis was estimated using the CINeMA (Confidence In Network Meta-Analysis) framework and online application [15].
RESULTS
Ten randomized trials conducted between 2010 and 2022 were selected. Of them, 4 studies compared the iTNT and CRT groups; 2 studies compared the cTNT and CRT groups; 3 studies compared the mTNT and CRT groups; and 1 study compared the iTNT and cTNT groups (Fig. 2).
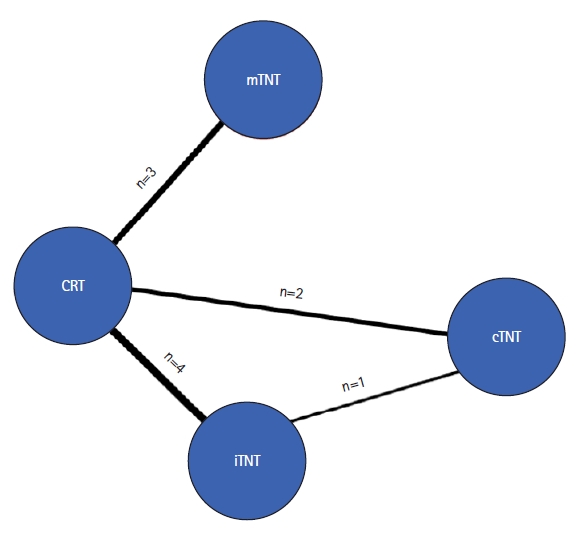
Network plot of relevant studies. CRT, chemoradiotherapy; TNT, total neoadjuvant treatment; cTNT, long-course CRT (50–54 Gy) followed by consolidation chemotherapy; iTNT, induction chemotherapy followed by CRT (50–54 Gy) and capecitabine; mTNT, short-course radiotherapy (25 Gy) followed by consolidation chemotherapy
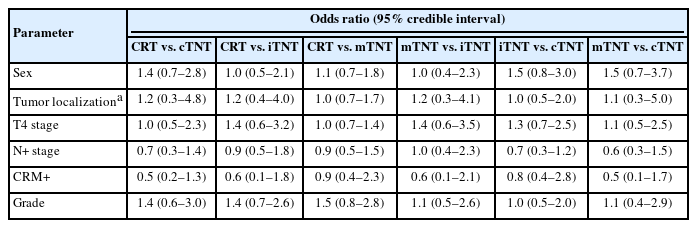
The total number of included patients was 2,719 (1,758 men, 64%; 961 women, 36%). The patients were distributed among the groups as follows (Supplementary Table 1) [16–25]: iTNT, 506 patients (18%); cTNT, 230 patients (9%); mTNT, 792 patients (29%); and CRT, 1,191 patients (44%).
Comparison of the basic clinical and morphological characteristics
The groups were analyzed according to the following characteristics: sex, tumor localization (low, mid, and upper rectum), T and N stages, involvement of the circumferential resection margins, and tumor differentiation. The data of the studies included in the meta-analysis did not demonstrate systematic selection bias of patients according to the basic clinical and morphological characteristics of the participants (Table 1).
Pathologic outcomes
Fig. 3 shows a forest plot of the results from the Bayesian network meta-analysis of the enrolled studies. Based on these results, the SUCRA was calculated. Statistically significant differences were detected in the rate of pCR when TNT groups were compared with the CRT regimen. Thus, the pCR rate was 20% (OR, 1.77; 95% CrI, 1.08–2.75) in the iTNT group, 21% (OR, 1.89; 95% CrI, 1.24–2.73) in the mTNT group, and 22% (OR, 2.59; 95% CrI, 1.29–4.90) in the cTNT group compared with 12% in the CRT group (Fig. 3). Furthermore, cTNT (Fig. 4) demonstrated the highest SUCRA probability (89%), followed by modified TNT (59%), TNT with induction chemotherapy (50%) and CRT (0.7%). Thus, the SUCRA ranking revealed that the TNT regimen has a better chance of pCR than standard CRT.

Forest plot of the pathologic complete response rate. (A) Forest plot. (B) League table. OR, odds ratio; CrI, credible interval; CRT, chemoradiotherapy; TNT, total neoadjuvant therapy; cTNT, long-course CRT (50–54 Gy) followed by consolidation chemotherapy; mTNT, short-course radiotherapy (25 Gy) followed by consolidation chemotherapy; iTNT, induction chemotherapy followed by CRT (50–54 Gy) and capecitabine.

Ranking plots for the efficacy of the comparison of outcomes among different neoadjuvant regimens. (A) Pathologic complete response. (B) Overall survival. (C) Local recurrence rate. (D) Metastasis rate. SUCRA, surface under the cumulative ranking curve. CRT, chemoradiotherapy; TNT, total neoadjuvant treatment; cTNT, long-course CRT (50–54 Gy) followed by consolidation chemotherapy; iTNT, induction chemotherapy followed by CRT (50–54 Gy) and capecitabine; mTNT, short-course radiotherapy (25 Gy) followed by consolidation chemotherapy.
There were not any significant differences in the R0 resection rate, which were 86% (414 of 478) in the iTNT group, 86% (151 of 175) in the cTNT group, 82% (644 of 782) in the mTNT group, and 81% (899 of 1,109) in the CRT group (Fig. 5).

Forest plot of the R0 resection rate. (A) Forest plot. (B) League table. OR, odds ratio; CrI, credible interval; CRT, chemoradiotherapy; TNT, total neoadjuvant therapy; cTNT, long-course CRT (50–54 Gy) followed by consolidation chemotherapy; iTNT, induction chemotherapy followed by CRT (50–54 Gy) and capecitabine; mTNT, short-course radiotherapy (25 Gy) followed by consolidation chemotherapy.
Long-term outcomes
A network meta-analysis of OS, LRR, and MR was undertaken to assess how treatment influenced long-term outcomes. Only 4 studies reporting long-term outcomes were found in the literature. The results of CRT were compared with those of iTNT and mTNT. The number of patients was 287 (14%) in the iTNT group, 723 (37%) in the mTNT group, and 986 (49%) in the CRT group (Fig. 6). The median follow-up varied from 22 months (Fernández-Martos et al. [23]) to 8 years (Ciseł et al. [18]).
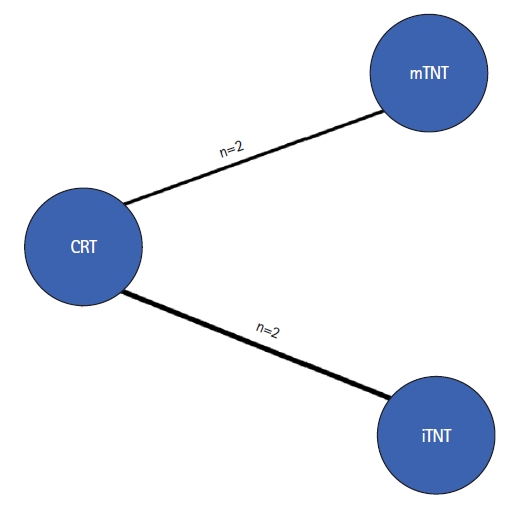
Network-plot of relevant studies with long-term outcomes. CRT, chemoradiotherapy; TNT, total neoadjuvant therapy; mTNT, short-course radiotherapy (25 Gy) followed by consolidation chemotherapy; iTNT, induction chemotherapy followed by CRT (50–54 Gy) and capecitabine.
The forest plot (Fig. 7) of the results of the network meta-analysis did not demonstrate any differences in long-term survival, though a trend for better outcomes was observed in the iTNT group with the SUCRA ranking (OS, 82%; LRR, 81%; MR, 71%) (Fig. 7). Thus, iTNT was identified as having the highest probability of being the best treatment, but the small number of published studies limited the analysis of long-term results.

Forest plots of long-term outcomes. (A) Overall survival. (B) Metastasis rate. (C) Local recurrence rate. RR, relative ratio; CrI, credible interval; CRT, chemoradiotherapy; TNT, total neoadjuvant therapy; iTNT, induction chemotherapy followed by CRT (50–54 Gy) and capecitabine; cTNT, long-course CRT (50–54 Gy) followed by consolidation chemotherapy; mTNT, short-course radiotherapy (25 Gy) followed by consolidation chemotherapy.
Consistency of the network and confidence in the estimates
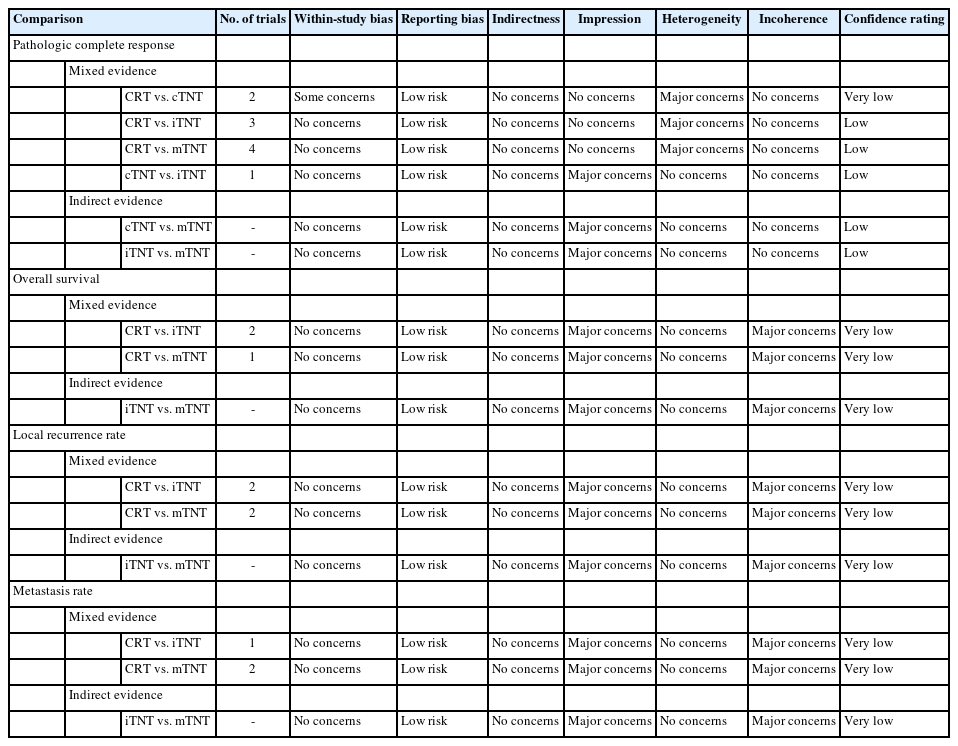
Inconsistency plots of the enrolled trials are shown in Fig. 8. The plot demonstrates the posterior mean deviance of each study for the consistency model (horizontal axis) and the unrelated meaneffects model (vertical axis), along with the line of equality. Significant inconsistencies were observed only between indirect evidence in the comparison of metastasis rates. The assessments of confidence in the estimates of pCR among different neoadjuvant regimes using CINeMA demonstrated very low confidence due to within-study bias. Furthermore, the estimates of long-term outcomes had low to very low confidence, owing to imprecision and heterogeneity (Table 2).

Plots showing the posterior mean deviance of the individual data points in the inconsistency model against their posterior mean deviance in the consistency model. Inconsistency model of pathology complete response (A), overall survival (B), local recurrence rates (C), and metastasis rates (D).
Methodological quality
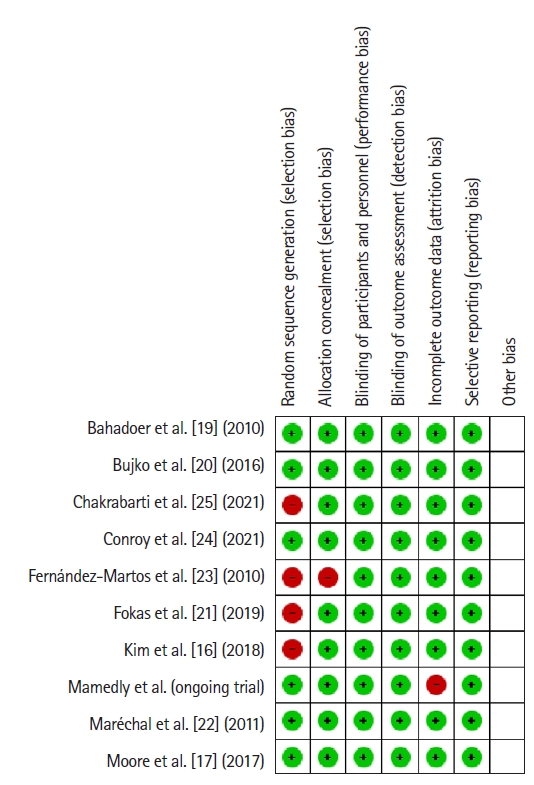
The patients or researchers in the studies included in the meta-analysis were not blinded in any of the publications. The details of randomization and allocation into groups were not available in some studies. A summary of the RoB is demonstrated in Fig. 9. The RoB for all enrolled studies is shown in Fig. 10.
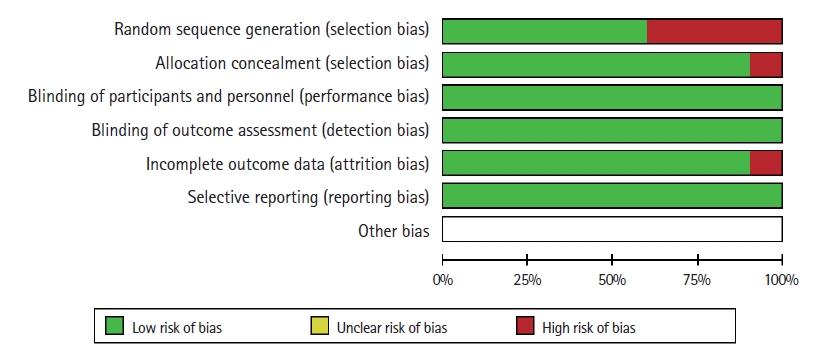
Overall assessment of the risk of bias. This diagram summarizes the bias risk for each included study as a generalized bias risk for the whole meta-analysis.
DISCUSSION
The combination of CRT and optimal surgical techniques has significantly improved the treatment outcomes of patients with nonmetastatic cancer of the mid and low rectum. This combined approach enables local control of disease to be achieved in most patients with stages II to III rectal cancer, although the 5-year OS rate in stage III does not exceed 70% to 76%, due to distant metastases. This fact confirms the importance of early administration of systemic treatment [1, 26, 27].
Standard protocols for the treatment of patients with stage III rectal cancer recommend systemic chemotherapy in the postoperative setting [28], though these recommendations are based on the results of clinical studies of adjuvant chemotherapy for colon cancer [29, 30]. The role of systemic chemotherapy in the treatment of rectal cancer has not been fully established [1, 31–33].
Another problem is the lack of unified study endpoints, which makes it difficult to analyze long-term outcomes. In this regard, pCR, defined as the complete absence of tumor cells in the surgical specimen, has been proposed as a surrogate predictor of long-term survival [3, 4].
The early administration of systemic chemotherapy in TNT can improve the treatment outcomes, increase the pCR rate, and reduce the risk of progression due to the elimination of micrometastases. Currently, there are 2 main approaches to TNT: iTNT and cTNT A variant of the second approach is a combination of a short-course RT and systemic chemotherapy, the so-called mTNT.
The present meta-analysis of randomized trials demonstrated that the addition of chemotherapy to radiation increases the pCR rate, which was 12% in the standard CRT group, 20% in the iTNT group, 21% in the mTNT group, and 22% in the cTNT group (Fig. 3). The TNT regimens showed the greatest efficacy if chemotherapy was administered in the waiting period after completing radiation therapy [34, 35].
The highest pCR rate was obtained in the cTNT group (22% vs. 12% in the CRT group; OR, 2.56; 95% CrI, 1.27–4.93) with statistically significant differences. In fact, in a randomized study by Kim et al. [16], where the rate of pCR was 13.6% (95% confidence interval [CI], 5.2–27.4) in the cTNT group versus 5.8% in the control group (95% CI, 1.2–16.5; P=0.167), the authors reported that 10 of 55 patients (19%) had withdrawn from the study. The most common reason was the unwillingness of 6 patients to continue the treatment. The remaining patients from the TNT group and 2 patients from the control group were excluded due to violations of the study protocol.
Moore et al. [17], in a sample of 49 patients, achieved a 24% pCR rate in the TNT group versus 16% in the control group (P=0.49). However, the small number of recruited participants did not allow a statistically significant difference to be achieved between the 2 groups of patients. However, our network meta-analysis demonstrated the best chance of pCR in the cTNT group with an almost 89% SUCRA ranking.
More reliable data were obtained in a large, randomized study by Fokas et al. [21], who compared 2 different TNT regimens with induction (156 patients) and consolidation (150 patients) chemotherapy with 3 FOLFOX courses. The 25% rate (95% CI, 18%–32%) of pCR after consolidation chemotherapy confirmed the hypothesis about the advantage of TNT over standard CRT, where the rate of pCR was assumed to be 15% (P=0.001). The 17% rate (95% CI, 12%–24%) of pCR in the group with induction TNT was comparable to that of CRT (P=0.21).
TNT with an induction course of chemotherapy and modified TNT with a long course of consolidation chemotherapy also revealed high pCR rates: 21% in the mTNT group and 20% in the iTNT group. The RAPIDO (Rectal Cancer and Preoperative Induction Therapy Followed by Dedicated Operation) trial revealed a higher pCR rate in the modified TNT regimen, based on a short course of RT, than in conventional CRT (28% vs. 14%, P<0.0001). The interval between the end of radiation therapy and surgery was significantly longer in the experimental group, where patients received chemotherapy for 6 months after completing short-course RT [19]. However, in the Polish II study [20], where patients received short-course RT with 2 months of subsequent chemotherapy, no difference in the pCR rate was demonstrated: 16% (41 of 261) in the mTNT group versus 12% (30 of 254) in the CRT group (P=0.17).
The best result of pCR in the iTNT group was obtained in a large, randomized trial, PRODIGE (Partenariat de Recherche en Oncologie Digestive) [28], which compared TNT with 6 induction courses of FOLFIRINOX followed by RT (50 Gy) in 231 patients with conventional CRT (50 Gy) in 230 patients. A pCR rate of 28% was reached in the TNT group, compared with 12% after CRT (P<0.0001) [23]. Other trials comparing iTNT with less intensive induction chemotherapy did not confirm better results of iTNT.
The effectiveness of any neoadjuvant treatment for rectal carcinoma is largely reflected by the R0 resection rate, though this meta-analysis did not demonstrate significant differences in that regard. The R0 resection rate after conventional neoadjuvant CRT was 77%, and the best result (86%) among all TNT modes was obtained in the cTNT group, albeit without statistical significance (Fig. 6).
We performed a network meta-analysis of long-term outcomes to assess how pCR influenced survival. Because of a lack of data, standard CRT was compared only with iTNT and mTNT regimens. Our network meta-analysis showed trends for better OS, MR, and LRR in the iTNT group (Fig. 8). The small number of included studies did not allow the detection of any statistically significant differences among the regimens.
The RAPIDO trial demonstrated better results in terms of disease-related treatment failure at 3 years in the mTNT group (23.7% vs. 30.4% in the CRT group, P=0.019) and a lower rate of distant metastases (20% in the mTNT group vs. 27% after CRT, P=0.005). However, higher local rectal recurrence rates in the mTNT group (8% vs. 4% in the CRT group) have limited the potential benefits of the RAPIDO trial results. The longer interval between RT and surgery in the mTNT group might have led to a higher pCR rate. However, for patients without a complete or near-complete clinical response, the extended interval between randomization and surgery in the mTNT group compared with the standard of care group (median, 25.5 weeks [interquartile range, 24–27.9 weeks] vs. 15.9 weeks [interquartile range, 14.6–17.6 weeks]) might have been disadvantageous [35]. In contrast, the Polish II trial [20] demonstrated no differences in recurrence-free survival (53% vs. 52%, P=0.85). The rates of local recurrence and distant metastasis were 22% versus 21% (P=0.82) and 30% versus 27% (P=0.26), respectively. The OS rate was 49% in both groups (OR, 0.9; 95% CI, 0.7–1.1; P=0.4) when assessed at a follow-up period of 8 years.
This network meta-analysis was performed in accordance with the PRISMA recommendations, and the methodological quality of included studies was evaluated in accordance with the Cochrane manual. We obtained statistically significant differences, with higher pCR rates in the TNT groups than in the CRT groups. However, in the analysis of the long-term results, the higher pCR rates did not increase long-term survival rates due to a lack of data. Another problem is the median follow-up reported in the presented studies, which ranged between 1.5 and 8 years. Furthermore, no long-term results of TNT with consolidation chemotherapy were published. The results may also be overstated, because the CINeMA evaluation according to GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) gave low and very low confidence levels for the study comparisons.
In conclusion, TNT has a demonstrable pCR advantage over CRT. The highest rate of pCR was obtained in the TNT groups, where systemic treatment was prescribed after completing radiation therapy. Further well-designed randomized trials with the assessment of survival as the primary endpoint are necessary to reveal long-term advantages of the TNT approach in the treatment of rectal cancer.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Acknowledgments
The authors thank Zaman Mamedli, Dmitriy Kuzmichev, and Andrey Polynovskiy of N.N. Blokhin National Medical Research Center of Oncology (Moscow, Russia) for kindly sharing the information from their unpublished trial.
Author contributions
Conceptualization: SS, A Ponomarenko, ER; Data curation: SS, A Polynovskiy; Formal analysis: SS, A Ponomarenko; Investigation: SC, MA, ZM, DK; Methodology: A Ponomarenko; Supervision: ER; Validation: A Ponomarenko, ER; Visualization: SS, A Ponomarenko, ZM, A Polynovskiy; Writing–original draft: SS, SC, MA, DK; Writing–review & editing: ER. All authors read and approved final manuscript.
Supplementary materials
Supplementary Table 1. Characteristics of the randomized trials
Supplementary materials are available from https://doi.org/10.3393/ac.2022.00920.0131.
Characteristics of the randomized trials