Neuroendocrine carcinoma associated with chronic ulcerative colitis: a case report and review of the literature
Article information
Abstract
Adenocarcinoma is a common histological type of ulcerative colitis-associated cancer (UCAC), whereas neuroendocrine carcinoma (NEC) is extremely rare. UCAC is generally diagnosed at an advanced stage, even with regular surveillance colonoscopy. A 41-year-old man with a 17-year history of UC began receiving surveillance colonoscopy at the age of 37 years; 2 years later, dysplasia was detected in the sigmoid colon, and he underwent colonoscopy every 3 to 6 months. Approximately 1.5 years thereafter, a flat adenocarcinoma lesion occurred in the rectum. Flat lesions with high-grade dysplasia were found in the sigmoid colon and surrounding area. The patient underwent laparoscopic total proctocolectomy and ileal pouch-anal anastomosis with ileostomy. Adenocarcinoma was diagnosed in the sigmoid colon and NEC in the rectum. One year postoperation, recurrence or metastasis was not evident. Regular surveillance colonoscopy is important in patients with long-term UC. A histological examination of UCAC might demonstrate NEC.
INTRODUCTION
Patients with long-standing ulcerative colitis (UC) are at a high risk of developing colorectal cancer [1, 2]. Surveillance colonoscopy is recommended to detect colorectal cancer at an early stage, especially for patients with pancolitis and left-sided colitis, who are at a high risk of UC-associated cancer (UCAC). If high-grade dysplasia (HGD) is detected, proctocolectomy is the first-choice elective surgery. The histology of UCAC is usually adenocarcinoma, with neuroendocrine carcinoma (NEC) being extremely rare. At the time of writing, only 14 cases of NEC associated with UC have been reported [3–5], including cases of colonic NEC [6–10] and rectal NEC [11–14]. In this article, we report the case of a patient with UC who developed an NEC of the rectum and review the clinicopathological features of the cases reported in the literature.
CASE REPORT
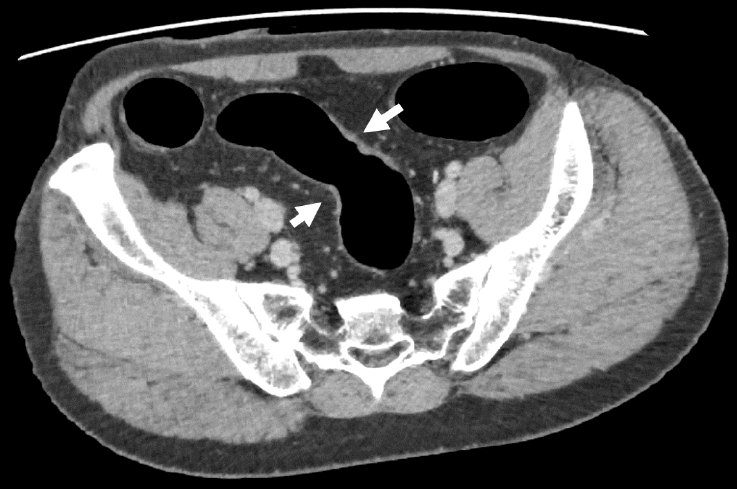
A 41-year-old man was diagnosed with UC in 1996 at a local clinic, which he attended for 10 years. The clinic had closed at the time of our investigation, and we were unable to obtain detailed records, but according to the information from a hospital that he attended for 10 years (1996–2006), he had never used any immunosuppressive drugs or biologic agents. Surveillance colonoscopy had been performed since 2013, and dysplasia was noted in a flat lesion in the sigmoid colon in 2019. Thereafter, repeat colonoscopies were performed every 3 to 6 months. At 1 year and 6 months after the initial diagnosis of dysplasia, endoscopy revealed HGD in the same lesion, and the patient was referred to our hospital for further investigation and treatment. Laboratory investigations revealed normal serum hemoglobin levels. The tumor markers carcinoembryonic antigen and CA19-9 were within the reference ranges, at 1.0 ng/mL (range, 0–5 ng/mL) and 6 U/mL (range, 0–37 U/mL), respectively. Repeat colonoscopy revealed a 0-IIa+IIc lesion in the rectum, and the biopsy revealed adenocarcinoma (Fig. 1A, B). Moreover, flat lesions were found in the sigmoid colon, and HGD was detected therein and in the surrounding area (Fig. 1C). Abdominal computed tomography scans revealed a focal thickening in the sigmoid colon, but no notable findings in the rectum (Fig. 2). No signs of distal organ or lymph node metastasis were observed, and the patient was diagnosed with cT2N0M0 rectal cancer. A laparoscopic total proctocolectomy and ileal pouch-anal anastomosis with ileostomy were performed. The postoperative period was uneventful.
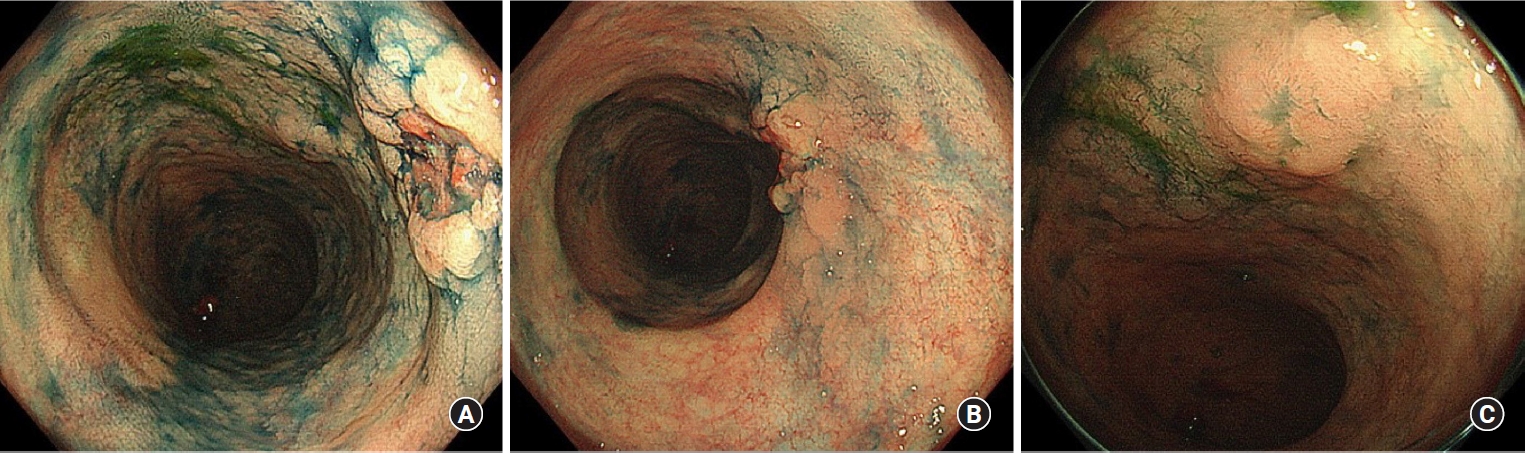
Endoscopic findings. (A) The 0-IIa+IIc lesion in the rectum. (B) Distant view of the 0-IIa+IIc lesion in the rectum. (C) Flat elevated lesion in the sigmoid colon.
Fig. 3A shows the macroscopic appearance of the excised specimen. Histopathology of the 0-IIa+IIc lesion in the rectum revealed NEC (Fig. 3B), whereas that of the preoperative biopsy revealed adenocarcinoma. Further, histopathology of the flat lesion in the sigmoid colon revealed adenocarcinoma (Fig. 3C, I), whereas that of the preoperative biopsy revealed HGD. HGD was diffusely present around both the NEC and adenocarcinoma (Fig. 3B, C, H, J). The 0-IIa+IIc lesion in the rectum showed mixed histological findings of NEC and well-differentiated tubular adenocarcinoma. The proportion of adenocarcinoma was 20%, and it did not meet the definition of mixed adenoneuroendocrine carcinoma. Immunohistochemically, the tumor cells were positive for synaptophysin and weakly positive for chromogranin A, and the Ki-67 index was >50%; thus, we diagnosed the tumor as NEC (pT3N0M0) (Fig. 3D–G). No evidence of metastasis from the NEC was observed in the lymph nodes; however, evidence of metastasis from the adenocarcinoma was noted (pT3N1bM0). Consequently, the patient was treated with capecitabine and oxaliplatin as postoperative adjuvant chemotherapy according to the treatment guidelines for colorectal cancer. The initial plan was for a 6-month course of postoperative adjuvant therapy; however, this therapy was discontinued after 4 cycles due to portal vein thrombosis. No sign of recurrence was noted 1.5 years after the operation.

Histological findings. (A) Macroscopic appearance of the excised specimen. (B) Magnified image of the 0-IIa+IIc lesion in the rectum, where neuroendocrine cell carcinoma (NEC) was detected; low high-grade dysplasia (HGD) was detected in the orange region, whereas NEC was detected in the black region. (C) Magnified image of the flat elevated lesion in the sigmoid colon, where adenocarcinoma was detected; HGD was detected in the orange region, and adenocarcinoma was detected in the black region. Adenocarcinoma was found adjacent to the NEC. (D) Hematoxylin-eosin (H&E) staining of the tumor revealing NEC (×400). (E) Immunohistochemical staining showing weak chromogranin A positivity (×400). (F) Immunohistochemical staining showing positive results for synaptophysin (×400). (G) Immunohistochemical staining showing Ki-67 >50% (×400). (H) H&E staining of the tumor showing that the NEC was adjacent to the HGD (×100). (I) H&E staining of the tumor showing adenocarcinoma (×100). (J) H&E staining of the tumor revealing that the adenocarcinoma was adjacent to the HGD (×100).
Ethics statement
This study was approved by the Institutional Review Board of the University of Tokyo (No. 3252-(15)). Written informed consent for publication of the research details and clinical images was obtained from the patient.
DISCUSSION
UC associated with NEC is a rare diagnosis. We searched PubMed for previously published case reports on UC associated with NEC by using the keywords “neuroendocrine carcinoma” and “ulcerative colitis” and found only 14 reported cases (Table 1). The median age of occurrence was 54 years, with a range of 35 to 77 years. The median duration of the disease from the initial diagnosis of UC to tumor development was 16 years, with a range of 11 to 30 years. All the cases were of the left-sided colitis type or pancolitis type, and only 2 patients were symptomatic.
Regarding medication, 10 patients (66%) had been treated with 5-aminosalicylic acid, 7 patients (46%) with corticosteroids, 1 patient (0.6%) with a biologic agent, and 3 patients (20%) with immunosuppressive agents. Our patient had received corticosteroid treatment for 10 years (1996¬–2006) after the diagnosis of UC and was in remission without any medication for the next 15 years. From a review of the literature, we could not identify any definitive factors related to UC-associated NEC.
One patient presented with abdominal pain due to cancerous bowel obstruction, while another patient presented with bloody stools. The remaining 13 patients were asymptomatic, and the diagnosis was made by surveillance colonoscopy.
Even when regular surveillance colonoscopy was performed once every 1 to 2 years, tumors were often detected at an advanced stage. Approximately 90% of the cases had lymph node or distant metastases (stage I, 0 cases; stage II, 1 case; stage III, 3 cases; stage IV, 7 cases; stage unknown, 4 cases). Even after surgery, recurrence and metastasis generally occurred within a short period, and the prognosis was extremely poor (survival period, 2–22 months). Therefore, it is important to consider the possibility of NEC in patients with long-term UC.
Although surveillance colonoscopy is effective for the early detection of UCAC, there is still no consensus on the time of initiation, frequency, and biopsy method. It has been reported that the standardized incidence rate of colorectal cancer in patients with UC is 5.7 times higher than that in the general population, and the standardized incidence rate is significantly higher in the pancolitis type (14.8 times) and left-sided colitis type (2.8 times) than in other disease types. In the present case, the lesion borders were difficult to discern, and NEC was detected postoperatively in an area where adenocarcinoma was detected preoperatively. The mechanism of NEC development and the relationship between NEC and UC has not yet been elucidated; moreover, in the latest European Crohn's and Colitis Organization guideline for inflammatory bowel diseases (IBDs) and malignancies, only carcinoid tumor (NET G2) is mentioned in association with IBDs. According to Iwabuchi and Sano [15], 4 pathways are involved in the mechanism of NEC development: differentiation from a preexisting adenocarcinoma, a preexisting carcinoid, nontumorous multipotential cells, and nontumorous juvenile endocrine cells. In the present case, the NEC occurred adjacent to the adenocarcinoma; therefore, the adenocarcinoma-NEC sequence was the most likely route of occurrence. Grassia et al. [4] suggested that neuroendocrine differentiation might take place from multipotential cells in the dysplastic epithelium since dysplasia was found in the adjacent mucosa in more than one-third of NEC cases, which makes it plausible that UC contributes to the development of NEC. Therefore, the detection of dysplasia is important, as it is a precancerous lesion of not only adenocarcinoma but also NEC. In patients with long-term UC, multiple biopsies from the surrounding mucosa (4 biopsies from around the tumor, if possible) should be obtained when a flat elevated lesion is found. A multicenter, retrospective observational study of surgical cases from 10 centers specializing in IBD surgery in Japan reported that cases detected by surveillance colonoscopy were diagnosed significantly earlier and had significantly better overall survival than those not detected by surveillance.
As the final pathological diagnosis was advanced colon cancer despite regular surveillance colonoscopy in this case, it is advisable to perform colonoscopy within a short timeframe and examine sufficient biopsy specimens if dysplasia is detected. Furthermore, if there is any doubt concerning the diagnosis, referral to a specialized facility and consultation with specialized pathologists are required.
A review of past cases revealed that many cases recur or metastasize within a short period after surgical treatment. The regimen for chemotherapy in patients with UC-associated NEC has not yet been established; however, in the literature, a chemotherapy regimen similar to that used for small-cell lung cancer is often selected. In the present case, as lymph node metastasis originated from the adenocarcinoma, capecitabine and oxaliplatin were administered in accordance with the postoperative adjuvant chemotherapy guidelines for colorectal cancer. A regimen for postoperative adjuvant chemotherapy should be established in future studies with more such cases.
Most cases of NEC in patients with UC become clinically evident abruptly after a few months from the last examination because they are proliferative and easily metastasize by frequent venous invasion [12]. Although NEC in patients with UC is extremely rare, we have highlighted the importance of including NEC in the differential diagnosis of colorectal tumors considering its aggressive behavior and poor outcomes. It is important to determine the risk factors for NEC given that current surveillance methods have limitations in detecting NEC at an early stage.
In conclusion, we reported a rare case of UC associated with NEC and showed that regular surveillance colonoscopy is important for patients with long-term UC. It is important to include NEC in the differential diagnosis of colorectal tumors in patients with UC. Further accumulation of data and studies on UC associated with NEC are necessary.
Notes
Conflict of interest
The authors have no conflicts of interest to declare.
Funding
None.
Author contributions
Conceptualization: Y Yokota, HA, SI; Investigation: TU; Project administration: Y Yokota, HA, HS, TS, Y Yoshioka, SA, Y Yokoyama, HM, SE, KM, KS, HN, TU, SI; Visualization: Y Yokota, HA, YN, HS, HN, TU, SI; Writing–original draft: Y Yokota, HA; Writing–review & editing: HS, TS, Y Yoshioka, YN, SA, HM, SE, KM, KS, HN, TS, SI. All authors read and approved the final manuscript.