Long-term bowel functional outcomes following anal sphincter-preserving surgery for upper and middle rectal cancer: a single-center longitudinal study
Article information
Abstract
Purpose
Despite advances in neoadjuvant chemoradiotherapy and anal sphincter-preserving surgery for rectal cancer, bowel dysfunction is still unavoidable and negatively affects patients’ quality of life. In this longitudinal study, we aimed to investigate the changes in bowel function with follow-up time and the effect of neoadjuvant chemoradiotherapy on bowel function following low anterior resection for rectal cancer.
Methods
In this study, 171 patients with upper or middle rectal cancer who underwent low anterior resection between 2012 and 2018 were included. Bowel function was assessed longitudinally with Memorial Sloan Kettering Cancer Center Bowel Function Instrument and Wexner scores every 6 months after restoration of bowel continuity. Patients with at least 2 follow-up visits were included.
Results
Overall, 100 patients received neoadjuvant chemoradiotherapy. Urgency, soilage, and fecal incontinence were noted within 24 months in the patients treated with neoadjuvant chemoradiotherapy. After 2 years of follow-up, significant bowel dysfunction and fecal incontinence were observed in the neoadjuvant chemoradiotherapy group. Low tumor level and neoadjuvant chemoradiotherapy were associated with delayed bowel dysfunction.
Conclusion
Neoadjuvant chemoradiotherapy in combination with low tumor level was significantly associated with delayed bowel dysfunction even after 2 years of follow-up. Therefore, careful selection and discussion with patients are paramount.
INTRODUCTION
The oncological outcomes of rectal cancer have improved over recent decades due to advancements in neoadjuvant concurrent chemoradiotherapy (CCRT), surgical techniques, total mesorectal excision (TME), and multidisciplinary team management [1, 2]. To avoid a permanent stoma, anal sphincter-preserving surgery (SPS) is frequently performed to preserve the anal sphincter, even in low-lying rectal cancers with colorectal or coloanal anastomosis [3]. However, this leads to the development of bowel dysfunction known as low anterior resection syndrome (LARS). The symptoms of LARS include urgency, fragmentation, clustering, incontinence for flatus and/or feces, and frequent bowel movements [4]. Reportedly, these symptoms most frequently present during the first postoperative year and stabilize over the long term [5]. Bowel dysfunction is associated with reduced quality of life (QoL) [6, 7]. Multiple risk factors are associated with bowel dysfunction, including low tumor level, old age, protective ileostomy, and radiation therapy [8]. Some studies have been focused on the effects of radiotherapy on bowel function and the anal sphincter, particularly the long-term observations that radiation therapy impairs bowel function and can cause scarring of the anal sphincter that results in increased fecal incontinence (FI) [9, 10]. Nowadays, the paradigm has shifted toward longitudinal studies of long-term outcomes regarding bowel function in patients with rectal cancer. Nevertheless, few studies in the literature have compared long-term longitudinal bowel functional outcomes between patients with rectal cancer who received CCRT and those who did not. The aim of this study was to assess the longitudinal trend of bowel function among patients with rectal cancer after SPS by reporting objective indicators at different postoperative intervals rather than a single time point, as well as to report any risk factors associated with delayed bowel dysfunction and FI with a focus on the effects of CCRT.
METHODS
Ethics statement
The study protocol was approved by the Institutional Review Board of Yonsei University (No. 4-2019-1098). Written informed consent was obtained from patients during the interviews for bowel function assessment. The study was reported in line with the Strengthening the Reporting of Cohort Studies in Surgery criteria [11] and was registered at ClinicalTrials.gov (identifier: NCT05339763).
Study design
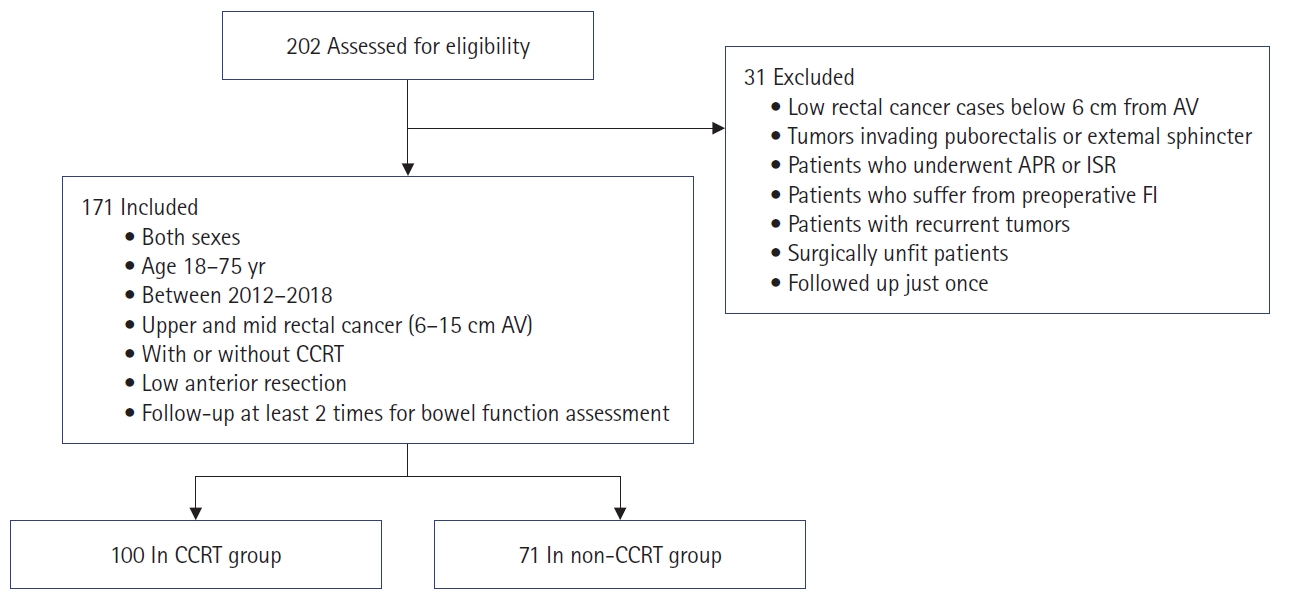
A total of 171 consecutively enrolled patients diagnosed with upper and middle rectal cancer were included in this retrospective longitudinal study, which was conducted between November 2012 and July 2018 in our colorectal surgery department. Data were collected prospectively.
Eligibility criteria
We included patients of both sexes, aged between 18 and 75 years, who had been diagnosed with upper or middle rectal cancer (6–15 cm from the anal verge [AV]) with or without preoperative CCRT, who underwent low anterior resection (LAR), and who underwent follow-up with at least 2 outpatient visits for bowel function assessment. Patients with recurrent tumors, surgically unfit patients, patients with tumors infiltrating the puborectalis muscle or external sphincter, those with preoperative FI, those who were followed up only once at the outpatient clinic, those with lower rectal cancer less than 6 cm from the AV, and those who underwent abdominoperineal resection or intersphincteric resection were excluded. We excluded patients with low rectal cancer less than 6 cm from the AV because such patients have comparatively poor bowel function, and thus poorer outcomes, because of the low tumor location and anastomosis level (Fig. 1).
Preoperative neoadjuvant chemoradiation
Preoperative long-course neoadjuvant 5-fluorouracil–based radiotherapy (45–50 Gy in 25–28 fractions) was provided to patients with locally advanced rectal cancer for tumor downstaging and, thereby, sphincter preservation. Surgery was performed 6 weeks after completion of chemoradiation.
Surgical procedure
The surgical procedure performed was LAR based on TME principles. Anastomosis was performed by either stapling (double stapling) after transabdominal specimen extraction through mini-laparotomy or via a hand-sewn technique with transanal specimen extraction. LAR was performed via open, laparoscopic, or robotic approaches. Covering loop ileostomy was sometimes used when the anastomosis level was low (as in middle rectal tumors), and these patients had higher rates of preoperative neoadjuvant radiotherapy. The ileostomy was retrieved at least 3 to 4 months postoperatively if no postoperative anastomotic complications arose. For those who had received adjuvant chemotherapy, ileostomy closure was postponed until adjuvant therapy completion.
Bowel function assessment follow-up
During follow-up at the outpatient clinic, a designated nurse interviewed the patients using bowel function assessment questionnaires at several time intervals between November 2015—when we began bowel function assessment at our institute using Memorial Sloan Kettering Cancer Center (MSKCC) and Wexner scores—and July 2019. Each patient was interviewed at least twice. Patients who underwent covering ileostomy in initial surgery due to lower anastomosis level were assessed for their bowel function after closure of the ileostomy, and all such patients had their ileostomies taken down.
The MSKCC and Wexner score questionnaires were used. The MSKCC questionnaire contains 18 questions divided into 3 subscales: a frequency subscale including 6 items, a dietary subscale with 4 items, and an urgency/soilage subscale with 4 items, along with 4 single items. Each subscale is scored by adding the scores for the component items, and the global score is the sum of all subscale scores. Finally, the total score is calculated by summing the global score and the scores for the 4 single items, with a maximum score of 90 [12].
The Wexner score questionnaire comprises 5 questions regarding solid, liquid, and gas incontinence; the use of a pad; and lifestyle alterations on a scale of 0 (no incontinence) to 20 (complete incontinence). The lower the score, the better the continence. In this study, FI was considered to be indicated by a Wexner score >8, based on the findings of a previous study in which a Wexner score of 8 was correlated with significant bowel-related QoL impairment [7]. We defined bowel dysfunction as being indicated by MSKCC score <65; this was based on the findings of a previous study in which good bowel function after resection for rectal cancer was defined as being indicated by MSKCC score >65 and poor bowel function by MSKCC score <65 [13].
We divided the patients into 2 groups (CCRT vs. non-CCRT) based on whether the patients received CCRT.
Statistical analysis
Statistical analysis was performed using IBM SPSS ver. 25.0 (IBM Corp). Quantitative data were described as mean±standard deviation or median and range, while qualitative data were presented as frequencies and proportions. The chi-square test was used for categorical variables. The Student t-test and the Mann-Whitney U-test were used to compare continuous variables. Risk factors for bowel dysfunction and FI were identified using binary logistic regression. P-values <0.05 were considered to indicate statistical significance.
RESULTS
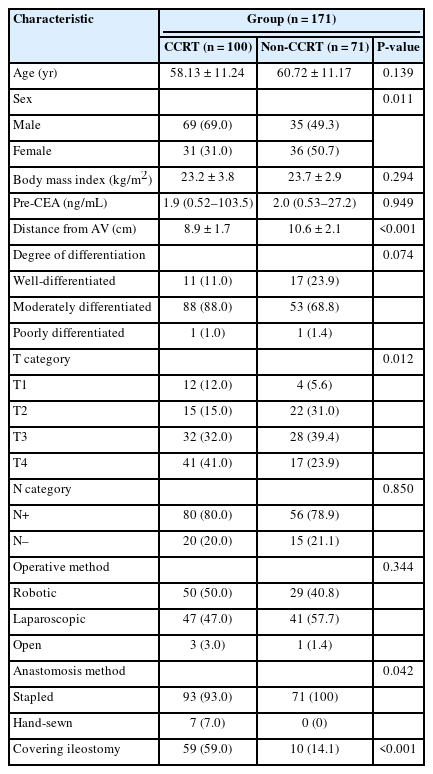
A total of 171 patients with rectal cancer were enrolled. The CCRT group comprised 100 patients, and the non-CCRT group included 71 patients. No significant difference was noted in age, body mass index, or preoperative carcinoembryonic antigen level between the 2 groups. The CCRT group contained more male patients than the non-CCRT group (P=0.011). The mean tumor distance from the AV was 8.9±1.7 cm in the CCRT group and 10.6±2.1 cm in the non-CCRT group (P<0.001). All patients in both groups underwent LAR. In the CCRT group, 59 patients (59%) were treated with covering ileostomy; unsurprisingly, this was significantly higher than the proportion in the non-CCRT group (P<0.001). The non-CCRT group contained more patients with stapled anastomosis than the CCRT group (P=0.042). Most procedures were performed in a minimally invasive fashion including robotic and laparoscopic approaches, and no significant difference in operative method was observed between groups (Table 1).
In terms of bowel function, during the first 18 months of follow-up, no significant difference was found between the 2 groups except in the dietary subscale during the first 6 months; throughout that period, the non-CCRT group demonstrated a significantly higher dietary subscale score than the CCRT group (P=0.013). Additionally, at 24 months of follow-up, patients treated with CCRT were observed to have higher scores for urgency, fecal soilage, and incontinence than those in the non-CCRT group (P=0.006). After 24 months of follow-up, the CCRT group exhibited more bowel dysfunction in the form of higher urgency, fecal soilage, and FI and lower dietary subscale scores than the non-CCRT group. We believe that these changes can be largely attributed to the delayed effects of CCRT (Table 2, Fig. 2).
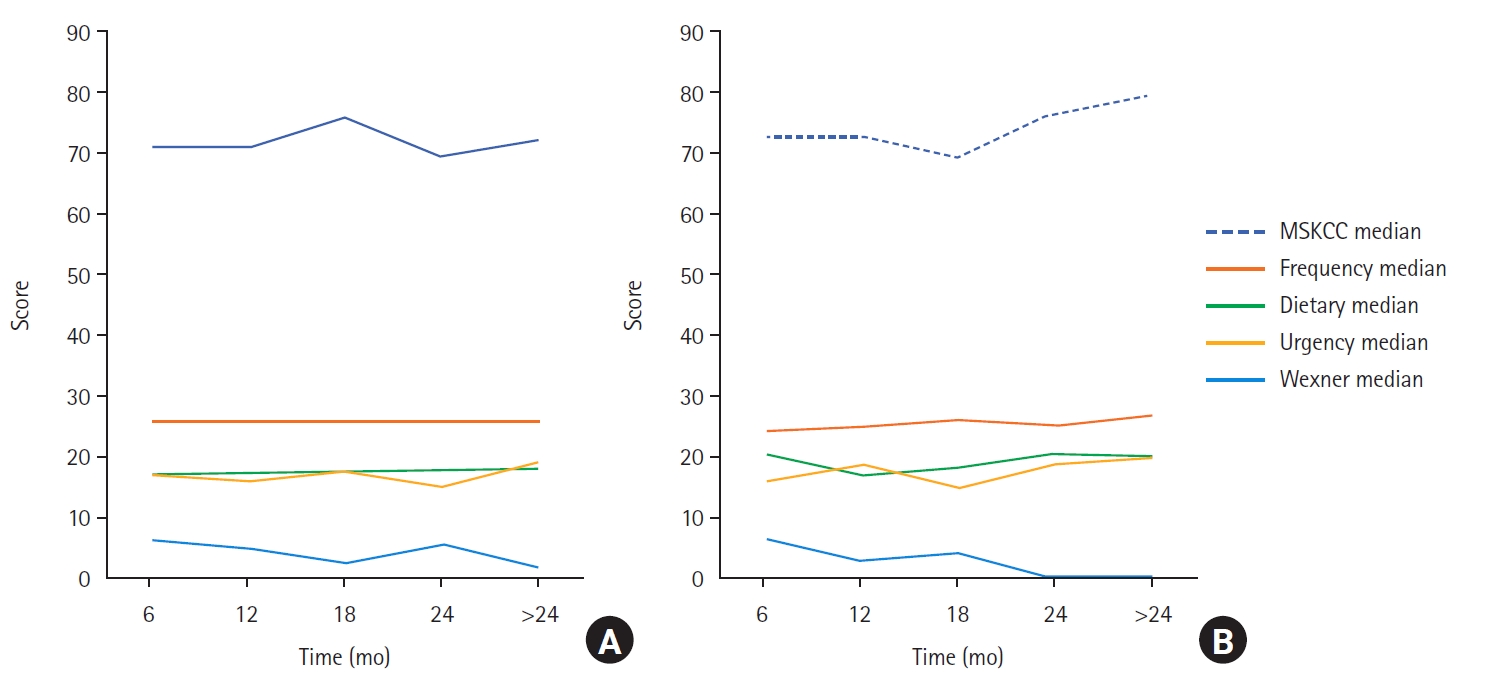
Changes in bowel function with time in both groups. (A) Concurrent chemoradiotherapy (CCRT) group. (B) Non-CCRT group. MSKCC, Memorial Sloan Kettering Cancer Center.
Generally, the overall median MSKCC score of the cohort improved with time, increasing from 71 to 80. The median frequency subscale also increased, from 25 to 26. The median dietary subscale was nearly static but increased from 17 to 19 during the last follow-up period, while the median urgency/soilage subscale score also increased from 17 to 19. Notably, the median Wexner score decreased over time from 6 to 0.
Risk factors associated with delayed bowel dysfunction and FI
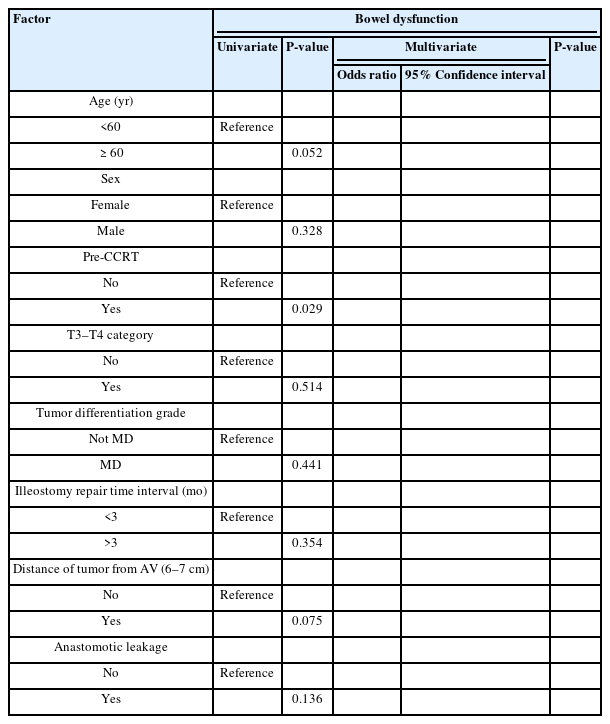
We performed univariate and multivariate analyses to identify risk factors associated with delayed bowel dysfunction and FI. The only risk factor for delayed bowel dysfunction (≥24 months) was CCRT, which was noted in the univariate analysis only (P=0.029). For delayed FI, CCRT was a risk factor in the univariate analysis alone, while tumor distance from the AV (6–7 cm) maintained its significance in the multivariate analysis as well (odds ratio, 0.166; 95% confidence interval, 0.028–0.989; P=0.049) (Tables 3, 4).
DISCUSSION
In recent years, the standard treatment for locally advanced rectal cancer has been CCRT combined with TME, which has demonstrated good oncological outcomes. However, adverse effects of radiotherapy on bowel function that negatively affect patient QoL have also been documented [14]. In the current study, we investigated the changes in bowel function following SPS for rectal cancer. We hypothesized that the bowel dysfunction and FI are due to effects of CCRT, especially a delayed impact. Furthermore, we raised multiple questions: When does bowel function usually improve? Is CCRT alone the cause of these adverse effects?
In this longitudinal study, patients underwent follow-up in 6-month intervals after surgery or ileostomy takedown to assess their bowel function using MSKCC and Wexner scores. Delayed bowel dysfunction and FI were observed in patients who had received CCRT at and after 24 months of follow-up.
The effects of bowel dysfunction are reportedly due not only to increased proximal colonic motility and reduction in the functional capacity of the neorectum, but also the associated anal sphincter dysfunction [15, 16]. Multiple cross-sectional and longitudinal studies have been conducted to investigate bowel dysfunction and its risk factors. In a longitudinal study of patients with low rectal cancer who underwent ultralow anterior resection (ULAR) with coloanal anastomosis, Cheong et al. [17] found that these patients still experienced major bowel dysfunction even 3 years after surgery, with risk factors of ULAR, male sex, old age, and adjuvant chemoradiotherapy. Additionally, Pieniowski et al. [18] observed no change in the prevalence of major bowel dysfunction over time, and approximately 50% of the patients who underwent SPS for rectal cancer experienced major bowel dysfunction 7 to 16 years after surgery. Findings of 2 other longitudinal studies demonstrated that 47.5% and 46% of patients experienced bowel dysfunction symptoms at 13.7 and 14.6 years, respectively [19, 20]. According to our results, the prevalence of bowel dysfunction symptoms and FI reached as high as 43% among patients treated with radiation at 24-month follow-up, which aligned with previously reported results [21, 22]. Studies have reported the incidence of bowel dysfunction to be approximately 19% to 52% [23]. In a recent meta-analysis by Croese et al. [24], the estimated prevalence of bowel dysfunction was 41%. In the present study, we observed a decline in symptoms of bowel dysfunction 1 year after surgery. Similar results were reported by Park et al. [13] in a cross-sectional study, in which they observed that a short follow-up period (≤1 year) was associated with major bowel dysfunction and FI. In recently published data from a retrospective cross-sectional study from our group investigating bowel function after resection of low-lying rectal cancer, we found partial improvements in bowel function 1 year after ileostomy takedown, and patients who underwent follow-up before 1 year still experienced major bowel dysfunction [25]. Furthermore, Perez et al. [26] reported similar findings of stabilized bowel function after the first year of follow-up.
Cheong et al. [17] reported ULAR as a risk factor for major bowel dysfunction during longitudinal follow-up with patients. Other studies have reported the same finding but in terms of the tumor height from the AV. Alavi et al. [8] pointed out that the nearer the tumor was to the AV (<6 cm), the worse the bowel function was in terms of the MSKCC score. In our study, we attempted to decrease the selection bias by excluding any low rectal cancer less than 6 cm from the AV, and the procedure performed was therefore LAR.
Many studies have highlighted the adverse effects of CCRT on bowel function, especially in long-term outcomes. Peeters et al. [10] found that patients who underwent radiation treatment experienced increased rates of FI, pad-wearing, anal bleeding, and mucus discharge. Furthermore, their overall satisfaction with bowel function was significantly lower than that of those who underwent surgery only. Pollack et al. [9] assessed the long-term effects of short-course radiotherapy on anorectal function using a questionnaire, anal manometry, and endorectal ultrasound; they reported that patients who had received radiation had significantly more frequent symptoms of FI and soiling, more bowel movements per week because of lower resting and squeeze pressures, and more scarring of the anal sphincter. Similar results were recently obtained by Quezada-Diaz et al. [27]; they observed that patients who had been exposed to radiotherapy alone had worse bowel function in terms of MSKCC score than those exposed to neoadjuvant chemotherapy alone. Results from a newly published randomized controlled trial also emphasize that CCRT and low anastomosis are independent risk factors of postoperative bowel dysfunction and decline in QoL [28].
In the present study, we observed that CCRT was associated with delayed FI after 2 years of follow-up in only the univariate analysis; meanwhile, tumor distance from the AV (<7 cm) was identified as a significant risk factor. Additionally, we observed the delayed deleterious effects of CCRT on bowel function in terms of MSKCC score.
Some studies have highlighted the effects of the covering stoma on bowel dysfunction. Walma et al. [29] demonstrated the risk of diverting ileostomy for FI and impairment of QoL after TME for rectal cancer. Furthermore, they mentioned that ileostomy reversal within 3 months was superior to delayed closure. Similar results were reported by Park et al. [13] in a cross-sectional study; they reported that ileostomy was associated with major FI and severe bowel dysfunction. We believe that stoma presence is associated with major bowel dysfunction because patients who undergo covering ileostomy initially present with tumors that are nearer the AV, receive more CCRT, and require a lower anastomosis level, which results in greater bowel dysfunction. However, in our study, we did not find the ileostomy closure time to be a risk factor for bowel dysfunction. A recently published longitudinal study [30] in a Scandinavian population addressed bowel function at 1 and 2 years of follow-up; this research indicated that major bowel dysfunction was common in young female patients and possibly persistent over time. This group of patients should be advised regarding early stoma closure to decrease such complications.
A strength of this study is that it revealed changes in bowel function over time in short 6-month intervals until more than 24 months of follow-up (constituting a longitudinal pattern). Additionally, it showed that combining CCRT and low anastomosis level to treat a tumor near the AV in a patient who undergoes LAR with a protective stoma affected bowel function in long-term follow-up.
Additionally, it is important to carefully select patients based on oncological safety. This is especially crucial in those with low-lying rectal cancer for whom CCRT is usually recommended. During the restaging period, if the tumor demonstrates a good response, SPS can be safely performed while considering a distal resection margin of more than 1 cm to prevent local recurrence. In other words, if the tumor still abuts the anal sphincter complex after CCRT, APR is a safer surgical treatment option than anal SPS. CCRT should also be reserved for locally advanced rectal cancers to minimize its deleterious effect on bowel function.
This study has the following limitations: retrospective design, lack of available anal manometric data, lack of QoL assessment, and selection bias due to loss to follow-up of some patients and heterogeneity of groups; however, we attempted to minimize this by excluding low rectal cancer cases (any case below 6 cm from the AV). Thus, further prospective studies are needed before drawing solid conclusions.
In conclusion, patients with CCRT showed significantly poor bowel function relative to patients without CCRT in this longitudinal study, even after 24 months from bowel restoration. CCRT with a low anastomosis level was a significant risk factor for delayed bowel dysfunction and FI. Therefore, preoperative counseling and patient selection are key for avoiding such complications that can compromise QoL.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: NKK; Data curation: AS; Formal analysis: all authors; Investigation: NKK; Methodology: NKK; Validation: NKK; Writing–original draft: AS; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Additional information
This study was presented at the International Colorectal Research Summit (ICRS) on September 6–8, 2019 in Seoul, Korea; at the 71st Annual Congress of the Korean Surgical Society on October 31–November 2, 2019 in Seoul, Korea; and at the 17th Scientific Conference of the European Society of Coloproctology (ESCP) on September 21–23, 2022 in Dublin, Ireland.