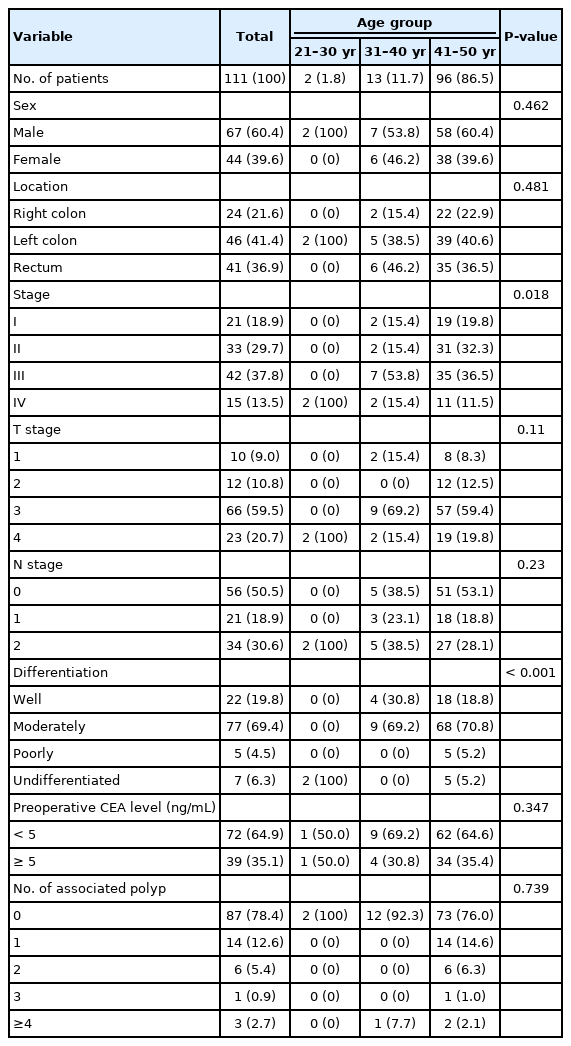
Clinicopathologic characteristics of early-onset colorectal cancer
Article information
Abstract
Purpose
The aim of this study was to analysis of the clinicopathological characteristics and prognosis of colorectal cancer (CRC) under the age of 50 years.
Methods
Between January 2009 and December 2018, 1,126 primary CRC patients were included from National Health Insurance Service Ilsan Hospital. The patients were divided into group 1 (n=111, ≤50 years) and group 2 (n=1,015, >50 years). The clinicopathologic features and prognostic outcomes were compared. In addition, to analyze whether there were any differences of those characteristics in 3 groups, patients aged under 50 years were divided into their 20s, 30s, and 40s.
Results
Group 1 had a slightly higher distribution in the left colon and rectum, lower T stage I and higher T stage IV rate, and a significantly higher distribution in stage N2 than group 2 (30.6%:16.3%, P<0.001). Poor histological differentiation of tumors was significantly high in group 1 (P=0.003). The 5-year survival rate for those in their 30s (69.2%) and 40s (91.6%) was higher than those in their 20s who died immediately after surgery (P<0.001). The 5-year disease-free survival rate was also confirmed to be meaningful for each age group, with 0% in their 20s, 53.8% in their 30s, 79.2% in their 40s (P<0.001).
Conclusion
Although the age was not an independent prognostic factor for overall survival in this study, the early onset group of CRCs is more advanced at the time of diagnosis and has a more aggressive histologic type.
INTRODUCTION
Colorectal cancer (CRC) is one of the most common cancers in Western Europe and the United States, and its incidence has increased significantly in East Asian countries such as Korea [1]. The onset of disease in genetic CRCs is known to be about 45 years old [2], but sporadic CRCs are mostly patients aged 65 years or older. However, recent reports show an increasing incidence among young people [3, 4].
Because CRC occurs from several genetic mutations, sporadic CRCs are regarded primarily as diseases affecting older people over 60 years. However, the biological behavior of cancer cells in young patients is known to be more aggressive than in older patients, so young age itself has been assumed to be a factor associated with a worse prognosis [5, 6]. To date, several studies have been conducted on the assumption that sporadic CRC biological behavior in older patients may differ from that of younger patients, with some authors reporting advanced tumor stage [5], high rates of poorly differentiated cancer [6], and short survival rates in younger patients [7-9]. However, a recent study of many patients reported no significant difference in survival rates between young and old groups [10, 11]. There is little research on young CRC patients in South Korea, so it is necessary to check the status of CRC according to age, and research on whether the patient’s age affects prognosis in sporadic CRC in the future may help improve the treatment strategy in the future. In this study, patients with sporadic CRCs were divided based on the age of 50 years proposed by Siegel et al. [3] in 2020 to reveal the difference in clinical and pathological characteristics and prognosis between the 2 groups.
METHODS
Patients and clinical data
Among primary CRC patients who underwent surgery at the National Health Insurance Ilsan Hospital (Goyang, Korea) between January 2009 and December 2018, 1,126 cases, excluding genetic CRC, were included in the study. Data on the clinical and pathological characteristics of the CRC were collected from clinical records and pathological findings, and the patient’s survival was confirmed by the final hospital visit date and direct contact. Of the total 1,126 people, 66.2% had colon cancer and 33.8% had rectal cancer. The median of their tracking period was 36.3 months, and the tracking period was at least 0.1 months up to 120.3 months (average, 42.0 months). This study protocol was approved by the Institutional Committee of National Health Insurance Ilsan Hospital (No. 2019-09-029) and the informed consent was waived by the Committee.
Patients were divided into 2 groups based on the age of 50 years; patients aged ≤ 50 years were divided into group 1 (n= 111), and patients aged > 50 years were divided into group 2 (n= 1,015). Differences between the 2 groups on sex, location of tumor, clinical stage, T stage, N stage, histologic differentiation, preoperative carcinoembryonic antigen (CEA) level, the number of polyps, and so on were compared according to clinical and pathological characteristics. In addition, those aged ≤ 50 years were divided into 20s, 30s, and 40s to analyze whether there were any differences in the above characteristics among the 3 groups.
Statistical analysis
The independent t-test and chi-square test were conducted to check the difference in the mean and frequency of covariates by type of CRC. The 5-year survival rate was calculated by utilizing the Kaplan-Meier curve. The survival rate was calculated according to the T (cerebrated tumor) and N (transfer to lymph node) stages. The statistical program used in the analysis is SAS ver. 9.3 (SAS Institute, Cary, NC, USA).
RESULTS
Clinical and pathological properties of colorectal cancer
The ratios of male and female patients in groups 1 and 2 were about the same, nearly 6 to 4. Group 1 had a slightly higher distribution in the left colon and rectum, lower T stage I and higher T stage IV rate, and a significantly higher distribution in stage N2 (30.6% : 16.3%, P< 0.001) than group 2.
T stage and preoperative CEA levels showed no difference between the 2 groups. The number of polyps was meaningfully high in group 2 (P< 0.001), poorly differentiated tumors were significantly high in group 1 (P= 0.003) (Table 1).
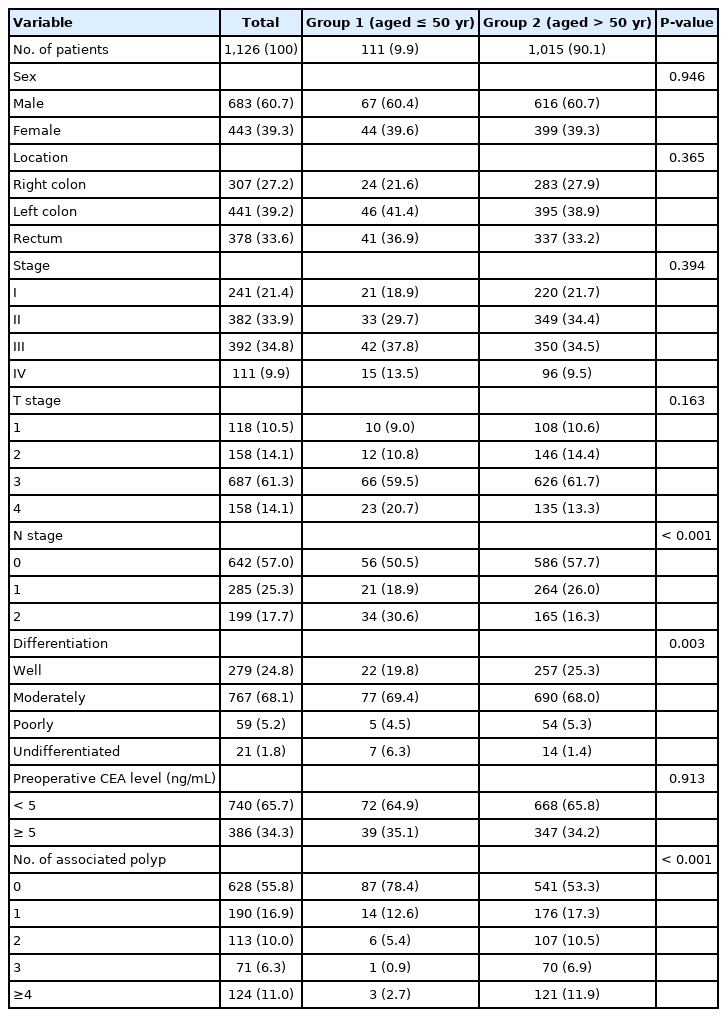
Comparisons of the clinicopathological features of the colorectal cancer patients between ≤50 and >50 years of age
Group 1 patients were divided into age groups and detailed analyses showed statistically significant levels of high stage and poor histologic differentiation of tumors in the group of 20s (Table 2), but the corresponding cases in the 20s were 2 cases, making them less statistically important (Table 2).
Survival rate analysis
The 5-year overall survival (OS) rate (group 1, 86.9%; group 2, 78.6%; P = 0.229) and 5-year disease-free survival (DFS) rate (group 1, 74.0%; group 2, 69.3%; P= 0.517) were not significantly different between the 2 groups (Fig. 1). The 5-year OS rate for those aged ≤ 50 years was significant in comparison with those in their 30s (65.6%) and 40s (91.9%) as both of those in their 20s died immediately after surgery (P< 0.001). The 5-year DFS rate was also confirmed to be meaningful for each age group, with 0% in their 20s, 52.5% in their 30s, and 79.0% in their 40s (P< 0.001) (Fig. 2). In addition, we analyzed the survival by dividing it into 3 groups based on the age of 40 and 65 years at the time of diagnosis. It showed that the 5-year OS rates under age 40 years were significantly lower ( ≤ 40 years, 62.5%; > 40 to ≤ 64 years, 86.5%; > 64 years, 75.5%; P= 0.001) (Fig. 3).
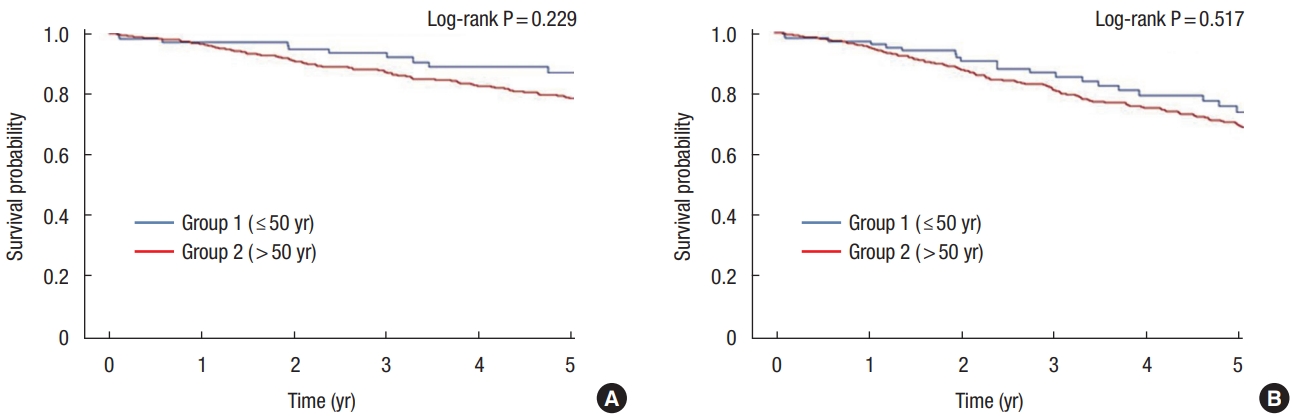
(A) Five-year overall survival rates in group 1 (aged ≤50 years, 86.9%) and group 2 (aged >50 years, 78.6%) (P=0.229). Five-year disease-free survival rate in group 1 is 74.0% and group 2 is 69.3% (P=0.517).

(A) Five-year overall survival rates according to the age at diagnosis (≤50 years) in 10-year increments (21–30 years, 0%; 31–40 years, 65.6%; 41–50 years, 91.9%; P<0.001). (B) Five-year disease-free survival rates according to the age at diagnosis (≤50 years) in 10-year increments (21–30 years, 0%; 31–40 years, 52.5%; 41–50 years, 79.0%; P<0.001).

Five-year overall survival rates according to the age at diagnosis (<40 years, 62.5%; 41–64 years, 86.5%; <65 years, 75.7%; P=0.001).
The results of the analysis of the 5-year OS rate and the 5-year DFS rate of the tumor, T and N stages, and preoperative CEA levels for the entire CRC patient showed significant differences in survival rates (P< 0.001) (Fig. 4).
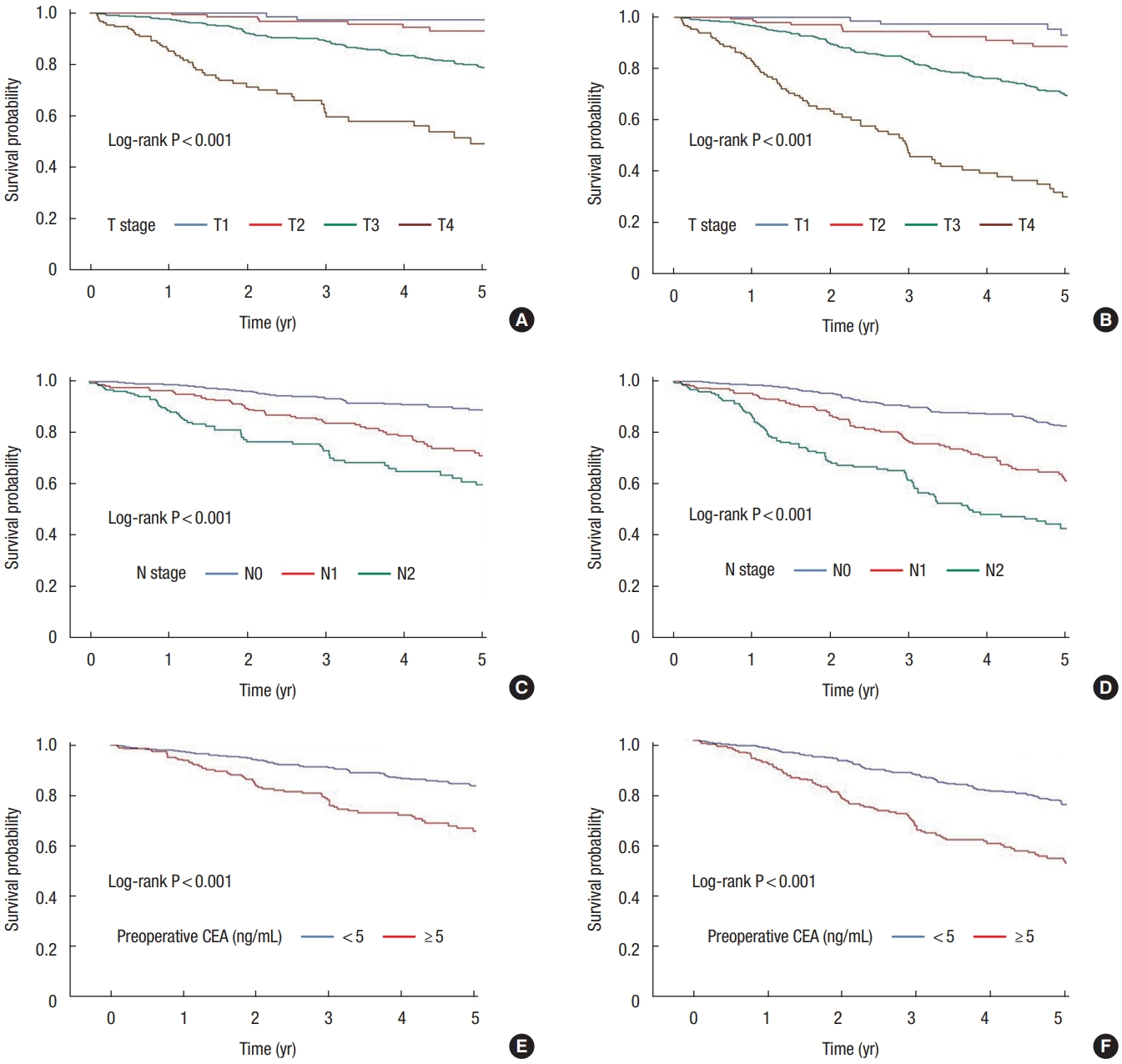
(A) Five-year overall survival (OS) rates according to the T stage (T1, 97.2%; T2, 93.0%; T3, 78.8%; T4, 49.2%; P<0.001) (B) Five-year disease-free survival (DFS) rates according to the T stage (T1, 93.0%; T2, 88.6%; T3, 70.0%; T4, 30.2%; P<0.001). (C) Five-year OS rates according to the N stage (N0, 89.0%; N1, 71.1%; N2, 59.6%; P<0.001). (D) Five-year DFS rates according to the N stage (N0, 82.5%; N1, 62.1%; N2, 42.5%; P<0.001). (E) Five-year OS rates according to the preoperative carcinoembryonic antigen (CEA) level (<5 ng/mL, 83.8%; ≥5 ng/mL, 65.9%; P<0.001). (F) Five-year DFS rates according to the preoperative CEA level (<5 ng/mL, 75.1%; ≥5 ng/mL, 53.3%; P<0.001).
DISCUSSION
The characteristics of the sporadic CRC’s biological behavior patterns in young patients are still unclear [12]. Studies of young CRC patients have been subject to several different criteria because of the ambiguous definition of young age [6]. Some studies considered young patients below the age of the CRC screening test, while others classified patients 10 years younger than the age of the CRC screening test as young patients based on treatment guidelines [13, 14]. Thus, further studies of the CRC in consideration of age in the future need to be conducted, and the basis for which age can be defined as young.
This study found that primary CRC patients under 50 years of age had a higher frequency of occurrence on the left-sided location, more advanced stage than those over 50 years of age, poor histological differentiation, and a more advanced nodal stage at diagnosis. In addition, group 1 was classified by age, and as a result of a detailed analysis, the prognosis was poor and histological differentiation was significantly low in the group in their 20s.
Contrary to our results, some studies showed no difference in survival even with younger age [15]. CRC patients were divided into their 30s, 40s, and 50s, and unlike our results, there was no difference in 5-year OS and DFS. However, there is a previous paper, which has a similar result to our research [16]. This study analyzed those patients diagnosed with colon cancer at a young age, especially at the age of 20 to 34 years, who showed a higher stage and worse prognosis.
Rho et al. [13] compared the CRCs of the younger (aged 18–44 years) and the older (aged ≥ 45 years) group and reported that CRC patients in the younger group showed more aggressive biomedical behavior, although treatment patterns and survival results are similar to those of the older group. Segev et al. [17] proposed a new CRC screening protocol different from before. They reported that the left CRC prevailed in both groups when classifying patients based on the age of cutoff at age of 50 years, and the authors proposed that the CRC screening be conducted from the age of 40 years, next follow-up was required at age of 45 years for negative findings, and colonoscopy was recommended at age of 50 years.
Ahnen et al. [6] reported that young patients (aged 20–40 years) were found to have a lower OS rate than the older patients (61.5% vs. 64.9%, P = 0.02). However, another study reported that the 5-year OS rate and the 5-year DFS rate were similar in the 2 groups compared to the age of 50 years [18]. As a result of comparing the survival rate by dividing it into 3 groups based on the age of 40 and 65 years, the OS rate for 5 years was significantly lower in the group under the age of 40 years. Therefore, the authors suggested that rediscussion and analysis of the age criteria of the CRC young patient group are needed. In our study, the differentiated canceler in the 20s group was 2 cases, and all died immediately after surgery. For this reason, when group 1 was classified by age (20s, 30s, 40s), the 5-year OS rate and the 5-year DFS rate showed significant differences by age group. However, the number of patients in their 20s is too small to give statistical significance.
Previous studies report that the systematic differentiation of tumors is not an independent predictor that affects survival rates [19]. An example of poor organizational differentiation suggested that the OS was worse because it was a cancerous disease that had already progressed at the time of diagnosis. The National Cancer Database in the United States reported that the young military’s CRC had a high frequency of mucous membranes and phosphorus cell species [6], but the cause of this difference in organizational differentiation is still unknown. In particular, young patients are likely to have been diagnosed with CRC of progressive diseases because the symptoms were not recognized or evaluated through examinations.
Until now, there has been a lack of sufficient understanding of the survival outcomes as well as the criteria for defining young adult CRC patients. CRC has heterogeneous characteristics, and researchers have generally focused on older patients with sporadic CRCs and younger patients with genetic CRCs. However, the proportion of young patients with sporadic CRCs is increasing, and most young patients are diagnosed after symptoms such as rectal bleeding or intestinal obstruction when the disease has already progressed [20]. This implies that, since these patients are not included in screening programs, they are often diagnosed in later stages of the disease [21]. In the case of sporadic CRC, it takes 5 to 10 years to change cancer after the screening test, so it would be beneficial to recommend the screening age group not to the 50s but before. Since most CRC patients tend to be examined and diagnosed after the recommended age of the screening, studies of patients with multiple sporadic CRCs should identify common characteristics of young patients and reflect them in terms of treatment and prognosis as well as in the future diagnosis. In Korea, the incidence of colon cancer has been decreasing in recent years in both 50 and under 50 years. For this reason, it can be estimated that in Korea, which has excellent access to medical care, there is a high awareness of the risk of colon cancer, especially those in their 30s and 40s, and many colonoscopies and polypectomies are performed [22]. Therefore, it is thought that starting a screening test for CRC at a young age can reduce the incidence of CRC.
Recently, recommendations have been issued to lower the age of screening tests to lower the prevalence of CRC. The American Cancer Society has lowered their colonoscopy screening recommendation from 50 to 45 years of age in patients at normal risk for developing CRC [23]. In 2021, the U.S. Preventive Services Task Force also recommends screening for CRC in adults aged 45 to 49 years [24]. Others have posited using screening flexible sigmoidoscopy starting at age of 40 years, given the tendency for young individuals to develop left-sided primary tumors of the sigmoid colon or rectum [17, 25]. In addition, a multicenter cohort study argued that it was appropriate to perform colonoscopy screening from the age of 45 to 49 years in high-risk groups with factors such as men, smokers, and high blood pressure [26].
There are some limitations to this study. First, this study compared many young and elderly patients over a decade but was conducted in a single institution. Therefore, this cohort may not represent the characteristics of young patients in the CRC among the entire population. Second, in this study, only 9.9% belonged to a group of young patients, which differed from the previously reported distribution of the number of patients. In addition, the proposed CRC diagnosis and treatment guidelines have so far defined young and old based on the age of 50 years. However, since the age of 40 and 45 years may also differ significantly from the age of 50 years, a study is needed to compare the patient group on a 10-year basis for diagnosis rather than dividing it by a specific age in a large cohort. Third, studies on gene expression such as KRAS, NRAS, and MSI are required to explain the activated cell pathways of young CRC patients but were not included in the results of this study.
Because the study was retrospective, it excluded patients with genetic CRC based on medical records. However, since family history may have been overlooked in some patients, multiorganforward studies of various cohorts are needed to verify age, family components, and biomolecular characteristics during diagnosis. The results of these studies will help change treatment strategies.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.