Robotic natural orifice specimen extraction surgery (NOSES) for anterior resection
Article information
Abstract
Minimally invasive colorectal surgery is currently well-accepted, with open techniques being reserved for very difficult cases. Laparoscopic colectomy has been proven to have lower mortality, complication, and ostomy rates; a shorter median length of stay; and lower overall costs when compared to its open counterpart. This trend is seen in both benign and malignant indications. Natural orifice specimen extraction surgery (NOSES) in colorectal surgery was first described in the early 1990s. Three recent meta-analyses comparing transabdominal extraction against NOSES concluded that NOSES was superior in terms of overall postoperative complications, recovery of gastrointestinal function, postoperative pain, aesthetics, and hospital stay. However, NOSES was associated with a longer operative time. Herein, we present our technique of robotic NOSES anterior resection using the da Vinci Xi platform in diverticular disease and sigmoid colon cancers.
INTRODUCTION
Minimally invasive colorectal surgery is currently well-accepted, with open techniques being reserved for very difficult cases. Laparoscopic colectomy has been proven to have lower mortality, complication, and ostomy rates; shorter median length of stay; and lower overall cost [1] when compared to its open counterpart. This trend is seen in both benign [2] and malignant indications [3–5]. We have sought to transfer these tangible benefits of minimally invasive colonic resection to rectal resection using natural orifice specimen extraction surgery (NOSES), a technique that promises reduction in postoperative pain, wound complications, and incisional hernia risk of a transabdominal extraction.
We present illustrative scenarios of robotic NOSES anterior resection using the da Vinci Xi platform (Intuitive Surgical Inc) in benign diverticular disease and distal sigmoid colon cancer resection.
TECHNIQUE
Ethics statement
This study was approved by the Institutional Review Board of Peter McCallum Cancer Center. Written informed consent for publication of the research details and clinical images was obtained from the patient.
Preoperative workup
The patient is given mechanical bowel preparation commencing the day prior to surgery. Our regimen comprises 2 sachets of PicoPrep (Fresenius Kabi) and one sachet of Glycoprep-C (Fresenius Kabi).
Perioperative workup
The patient is placed in the Lloyd-Davies position. An indwelling urinary catheter is inserted. The rectum is washed out using 1,000 mL of warm cetrimide. Both mechanical and chemical deep vein thrombosis prophylaxis is given according to the institutional protocol.
Access
Optical abdominal entry is performed using the da Vinci 8 mm robotic port and 0° camera (Intuitive Surgical Inc) at 3 cm below the left subcostal margin in the midclavicular line. After pneumoperitoneum is achieved, additional 8-mm robotic ports are placed in a diagonal line, as shown in Fig. 1A. In cases of malignant disease, a 12-mm port is used in the right lower quadrant position (arm 4) to facilitate the use of a da Vinci SureForm stapler (Intuitive Surgical Inc). A 12-mm assistant port is placed in the right mid-clavicular line at the level of the umbilicus.
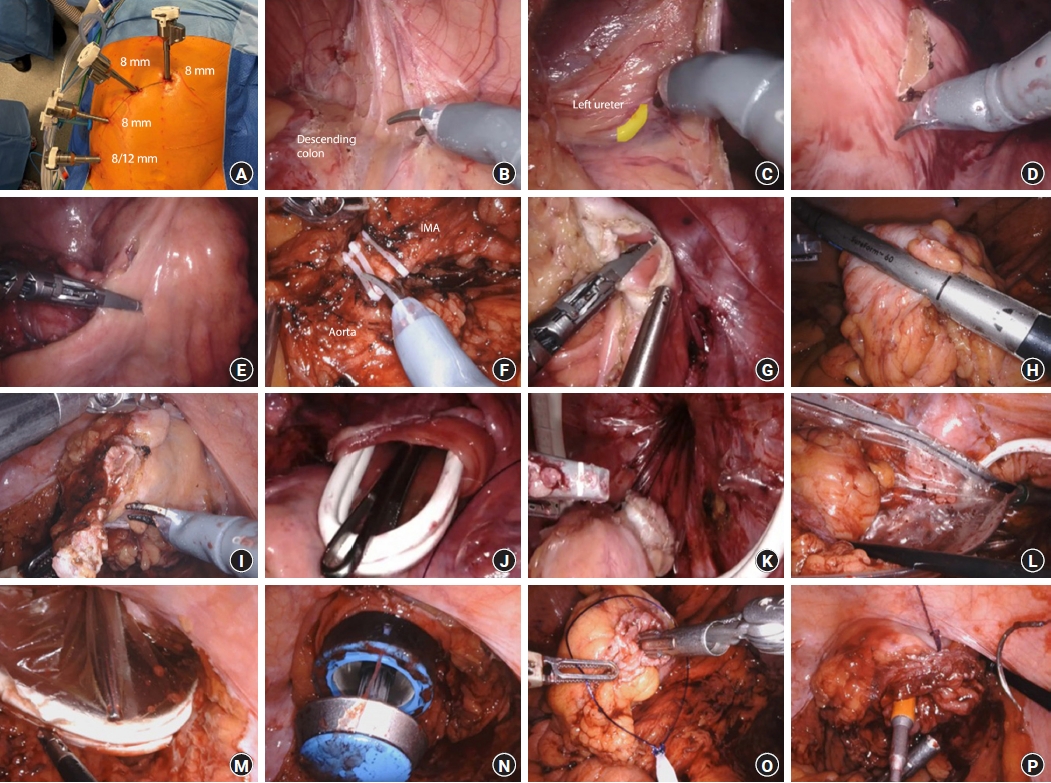
Robotic natural orifice specimen extraction surgery (NOSES) anterior resection technique. (A) Port placement. (B) Left colon mobilization. (C) Left ureter identification. (D) Scoring the mesentery of transection points. (E) Mesentery division with da Vinci Vessel Sealer (Intuitive Surgical Inc). (F) Vessels controlled with da Vinci Weck Hem-O-Lok (Intuitive Surgical Inc). (G, H) Bowel division. (I) Excision of distal staple line. (J) Transanal alexis retractor inserted. (K, L) Specimen removed transanally using an EndoCatch II bag (Medtronic). (M) Alexis rectractor removed. (N, O) Anvil inserted and secured. (P) Rectal stump purse string and anastomosis is performed.
Mobilization and specimen division
Mobilization of the descending and sigmoid colon is performed using monopolar scissors (Fig. 1B) with identification of the left ureter (Fig. 1C). It is important to mobilize the upper rectum to aid anchorage of an Alexis retractor (Applied Medical) and specimen extraction.
Mesenteric division
The proximal and distal transection points are marked by scoring the mesentery (Fig. 1D). For benign disease, the mesentery is divided close to the bowel wall using a da Vinci Vessel Sealer (Intuitive Surgical Inc) (Fig. 1E). For malignant disease, oncological principles are observed, with high ligation of the inferior mesenteric artery and vein using the da Vinci Weck Hem-O-Lok clip applicator (Intuitive Surgical Inc) (Fig. 1F). The mesentery is divided using the Vessel Sealer to the mesenteric edge of the bowel wall.
Bowel division
Tissue perfusion is assessed with indocyanine green using the da Vinci Firefly function (Intuitive Surgical Inc), and the point of bowel transection is determined. For benign disease, the proximal and distal bowel can be divided with robotic scissors or a Vessel Sealer (Fig. 1G). For malignant disease, the SureForm 60-mm stapler is used for proximal and distal division (Fig. 1H) to prevent tumor spillage. The rectal stump staple line is then excised to allow transanal specimen extraction and anastomosis (Fig. 1I).
Specimen extraction
An Alexis retractor is inserted transanally to aid passage of the specimen and protect the rectum and anal canal from potential tumor seeding (Fig. 1J). The specimen is retrieved transanally (Fig. 1K), using a 15-mm EndoCatch II bag (Medtronic) (Fig. 1L).
Intracorporeal anastomosis
The Alexis wound protector is removed (Fig. 1M). The anvil of a circular end-to-end stapler is the passed transanally into the abdomen (Fig. 1N) and secured to the proximal bowel using a 3-0 V-Loc (Medtronic) purse string suture and reinforced with an Endoloop PDS II (Ethicon) (Fig. 1O). The circular stapler is passed up the rectal stump to the rectotomy and the spike is deployed. The rectal stump purse string is created around the spike using 0 V-Loc and reinforced with Endoloop PDS II (Fig. 1P). Tensionfree anastomosis is performed and leak-tested. A diverting loop ileostomy may be required according to standard indications. Supplementary Video 1 outlines the aforementioned steps.
DISCUSSION
NOSES in colorectal surgery was first described in the early 1990s [6, 7]. Since then, there have been 41 published case series spanning both malignant and benign indications [8]. The transrectal and transanal route is more common than the transvaginal route, with the former comprising 30 series versus the latter with only 11 series [8]. Three recent meta-analyses comparing transabdominal extraction against NOSES concluded that NOSES was superior in terms of overall postoperative complications, recovery of gastrointestinal function, postoperative pain, aesthetics, and hospital stay [9–11]. However, NOSES was associated with longer operative time, likely due to increased technical complexity associated with an intracorporeal anastomosis [9–11]. There are specific technical differences for achieving natural orifice extraction when compared to transabdominal extraction that may be potential causes of concern and are addressed below.
An intraperitoneal enterotomy and the insertion of a stapler anvil into the abdominal cavity through a natural orifice are potential sources of contamination. Costantino et al. [12] and Wolthuis et al. [13] studied the bacterial positivity rate in peritoneal fluid culture and demonstrated that although NOSES had a higher risk of peritoneal contamination, there were no significant differences in clinical outcomes between the 2 groups. Methods to mitigate intraperitoneal bacterial contamination include perioperative prophylactic antibiotics, preoperative antibiotic bowel preparation, intraoperative peritoneal irrigation, and rectal washout prior to specimen extraction [14]. Furthermore, a recent meta-analysis by Liu et al. [10] concluded that surgical site infection was less common in the NOSES group than in the conventional laparoscopic surgery group, with similar intraabdominal collection rates between the 2 groups. This meta-analysis also demonstrated that NOSES had a lower leak rate (3.6% vs. 5.0%), and other studies have also reported consistent findings [15, 16]. The difference in the leak rate is likely a function of intracorporeal anastomosis, which has key technical advantages of less mobilization and less traction on the mesentery and blood supply, resulting in a more vascularized tension-free anastomosis.
Another important concern is oncological safety, arising from the need for colotomy/rectotomy and specimen extraction through a narrow natural orifice, which may lead to tumor cell seeding and implantation, respectively. In our practice, risk-mitigating steps taken include tumoricidal rectal washout, the insertion of a transanal Alexis retractor, and the utilization of a 15-mm specimen extraction bag. Reassuringly, other studies have confirmed that local recurrence after NOSES is comparable to that after a conventional laparoscopic approach [17], with comparable proximal and distal margin status and lymph node harvest [10]. Lastly, the 5-year disease-free survival and local recurrence rates are comparable between the 2 techniques [17].
With the adoption of colorectal NOSES, there arose a need for the standardization of terminology and techniques, as well as oversight of surgical indications and safety. Therefore, an international consensus statement on NOSES for colorectal cancer was published [14]. This document defines 10 different colorectal NOSES techniques, with various combinations of the type of resection and extraction site. Importantly, the consensus also provides a guideline on what is safe and feasible. In our experience, the patients who would be most suitable for transanal NOSES include patients with a maximum body mass index of 32 kg/m2, tumor diameter of 3 cm, and tumors that are in the sigmoid or proximal rectum. Female patients who have a tumor up to 5 cm in size may be appropriate for transvaginal NOSES.
One of the basic requirements for NOSES is an experienced laparoscopic surgeon due to the increased challenges of specimen extraction and gastrointestinal tract reconstruction [14]. These challenges can be partially offset by the benefits of a robotic platform such as the da Vinci Xi. The benefits of the high-definition 3-dimensional system, the ergonomic positioning of the surgeon, the instrument articulation with greater precision, and the absence of tremor might lead to higher accuracy, more precise dissection, a flatter learning curve, all potentially resulting in improved outcomes; not ignoring the fact that most robotic surgeons are themselves already accomplished laparoscopic surgeons. Further studies to evaluate the quality of life and functional outcomes are currently underway.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: PS; Data curation: TDP, TL, AR; Formal analysis: TDP, BO; Project administration: TDP, TL, AR, BO; Resources: RDP, PS, SKW; Software: TDP, SKW; Supervision: SKW, AGH, PS; Writing–original draft: TDP; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Supplementary materials
Technical steps during robotic anterior resection via natural orifice specimen extraction surgery (NOSES).
Supplementary materials are available from https://doi.org/10.3393/ac.2022.00458.0065.
