Small Bowel Obstruction After Ileal Pouch-Anal Anastomosis With a Loop Ileostomy in Patients With Ulcerative Colitis
Article information
Abstract
Purpose
Small bowel obstruction (SBO) remains a common complication after pelvic or abdominal surgery. However, the risk factors for SBO in ulcerative colitis (UC) surgery are not well known. The aim of the present study was to clarify the risk factors associated with SBO after ileal pouch-anal anastomosis (IPAA) with a loop ileostomy for patients with UC.
Methods
The medical records of 96 patients who underwent IPAA for UC between 1999 and 2011 were reviewed. SBO was confirmed based on the presence of clinical symptoms and radiographic findings. The patients were divided into 2 groups: the SBO group and the non-SBO group. We also analyzed the relationship between SBO and computed tomography (CT) scan image parameters.
Results
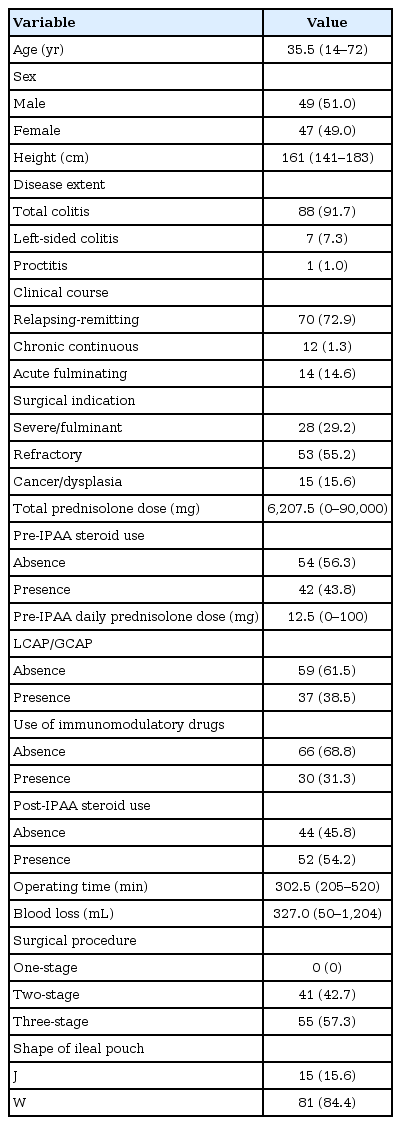
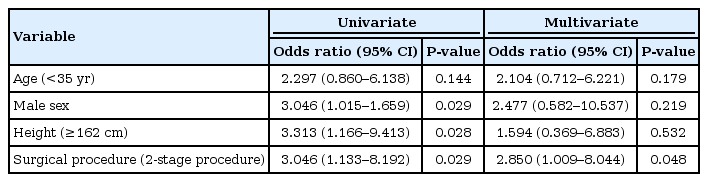
The study included 49 male and 47 female patients. The median age was 35.5 years (range, 14–72 years). We performed a 2- or 3-stage procedure as a total proctocolectomy and IPAA for patients with UC. SBO in the pretakedown of the loop ileostomy after IPAA occurred in 22 patients (22.9%). Moreover, surgical intervention for SBO was required for 11 patients. In brief, closure of the loop ileostomy was performed earlier than expected. A multivariate logistic regression analysis revealed that the 2-stage procedure (odds ratio, 2.850; 95% confidence interval, 1.009–8.044; P = 0.048) was a significant independent risk factor associated with SBO. CT scan image parameters were not significant risk factors of SBO.
Conclusion
The present study suggests that a 2-stage procedure is a significant risk factor associated with SBO after IPAA in patients with UC.
INTRODUCTION
Small bowel obstruction (SBO) is the most common complication in patients who undergo abdominal surgery. A prior report noted that SBO after abdominal surgery occurred in approximately 20% of the cases [1]. Menzies and Ellis [2] found a much higher proportion (93%) of patients with postoperative intra-abdominal adhesions at subsequent surgeries. These adhesions are the major cause of a SBO after surgery [3].
In 1978, Parks and Nicholls [4] reported that a total proctocolectomy (TPC) with ileal pouch-anal anastomosis (IPAA) was an effective treatment for patients with ulcerative colitis (UC). In 1980, Utsunomiya et al. [5] described a restorative proctocolectomy with an ileal J pouch for the treatment of patients with adenomatosis coli and UC. This type of surgery is a standard procedure for UC. In most cases, a 2- or a 3-stage procedure with a loop ileostomy is performed to prevent surgical complications, such as leakage of the anastomosis and pelvic abscess. However, the loop ileostomy itself may cause other complications, including skin irritation, prolapse, and bowel obstruction [6]. The complication rate of a loop ileostomy in abdominal surgery has been reported to be 5%–100% [7], and SBOs have frequently occurred in patients at a rate of 3%–9.9% [8-10]. These rates vary due to the differences in the lengths of follow-up for previous studies. The frequency of a SBO for UC surgery is comparatively higher than that for other abdominal surgeries because of the combined abdominal and pelvic dissection, need for multiple surgeries, and possibly a higher likelihood of septic complications [11]. The incidence of a postoperative SBO in UC surgery is estimated to be 6%–25.3% [11-18]. A SBO may lead to a decrease in the quality of life, as well as a longer duration of hospital stay. However, the risk factors associated with a SBO for UC surgery are not well known. Thus, the aim of the present study was to clarify the risk factors associated with a SBO after an IPAA with a loop ileostomy for patients with UC.
METHODS
We performed a retrospective review of data from hospital records. During the years 1999–2011, 96 patients underwent an IPAA for UC at Niigata University Medical and Dental Hospital. This study is retrospective observational study, carried out by the opt-out method of our institution. All patients underwent a rectal mucosectomy with a hand-sewn IPAA by open surgery. The IPAA has been usually performed in 1, 2, or 3 stages. In the 3-stage procedure, first, a subtotal colectomy with an end ileostomy/ascending colostomy and setting of the mucous fistula were performed. Subsequently, pouch surgery was performed as a second operation, and the stoma was closed as the third operation. In the 2-stage procedure, a TPC and an IPAA with an ileostomy were performed as the first operation, and the temporary loop ileostomy was closed 1–3 later as the second operation. The pouch shapes used for the IPAA included a W or a J pouch. The W pouch’s construction was used as the main procedure at our institute [19, 20]. A stapled pouch construction was also performed for a J pouch surgery. The J pouch’s construction was selected when performing an IPAA was difficult because of intra-abdominal adhesion, obesity, a narrow pelvis, and a small-bowel mesentery shortage. As a surgical approach, the 1-stage procedure is not performed at our institution. Therefore, a 2- or a 3-stage procedure was performed in all cases. We have performed the 2-stage operation for UC patients without factors such as poor general condition, poor nutrition, fulminant colitis, and a toxic megacolon. Hence, we selected the 3-stage procedure when the preoperative patient’s condition was severe. The optimal stomal placement site was preoperatively marked on the right lower quadrant abdominal wall. Closure of the loop ileostomy was performed 1–3 months after the IPAA.
In this study, a SBO was confirmed by both the presence of clinical symptoms, such as abdominal pain, nausea, vomiting, and no passage of flatus or stool, and abdominal radiographic findings. These clinical diagnostic criteria have been recommended by several studies [3, 11-13]. The patients were divided into 2 groups: the SBO and the non-SBO groups. Clinical variables, including age, sex, height, weight, body mass index (BMI), preoperative serum albumin level, clinical course, surgical indication, total prednisolone dose, pre-IPAA steroid use, pre-IPAA daily prednisolone dose, leukocytapheresis (LCAP)/granulocytapheresis (GCAP) treatment, use of immunomodulatory drugs, post-IPAA steroid use, operating time, blood loss, surgical procedure (2- or 3-stage procedure), stoma rotation, shape of the ileal pouch, and use of an adhesion barrier were compared between the 2 groups. We also analyzed the thicknesses of the abdominal rectus muscle and the subcutaneous fat and the size of the stoma at the skin or fascia level in the 2 groups by using computed tomography (CT) scan images. The thicknesses of the muscle and the fat were measured at the umbilical level by using CT scans. All patients who developed a SBO underwent conservative treatment, such as the cessation of an oral diet and/or the insertion of a transstomal decompression tube.
In this study, we analyzed the risk factors associated with a SBO after an IPAA with a loop ileostomy, namely, during the pretakedown period, in patients with UC. Statistical analyses were performed using the Mann-Whitney U-test or Fisher exact test. A multivariate logistic regression was performed to determine which significant factors from the univariate analysis remained significant. P-values of <0.05 were considered to be statistically significant. IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA) was used for the statistical analyses.
RESULTS
A total of 96 patients underwent an IPAA by open surgery for UC at Niigata University Medical and Dental Hospital between 1999 and 2011. The patients’ demographics are shown in Table 1. The median age of the patients was 35.5 years (range, 14–72 years), and the median follow-up period after the IPAA was 108 months (range, 53–192 months). Of these patients, 49 (51.0%) were male and 47 (49.0%) were female patients. The 1-stage procedure was not performed at our institution. A loop ileostomy was introduced in all cases.
A SBO occurred during the pretakedown of the loop ileostomy after the IPAA in 22 patients (22.9%). Moreover, surgical intervention was required for 11 patients (Fig. 1). In brief, closure of the loop ileostomy had to be performed earlier than expected. The cause of the SBO requiring surgery was an outlet obstruction in eight patients. In the remaining three patients, the cause of the SBO was considered to be intra-abdominal adhesion. Furthermore, in the 11 patients, the SBO improved with conservative treatment, such as the cessation of an oral diet and/or the insertion of a transstomal decompression tube (Fig. 2).
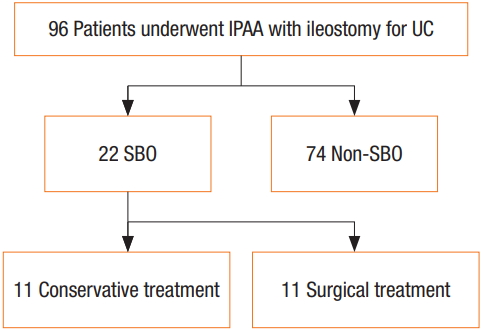
The outcomes for 96 patients who underwent ileal pouchanal anastomosis with loop ileostomy for ulcerative colitis. IPAA, ileal pouch-anal anastomosis; UC, ulcerative colitis; SBO, small bowel obstruction.
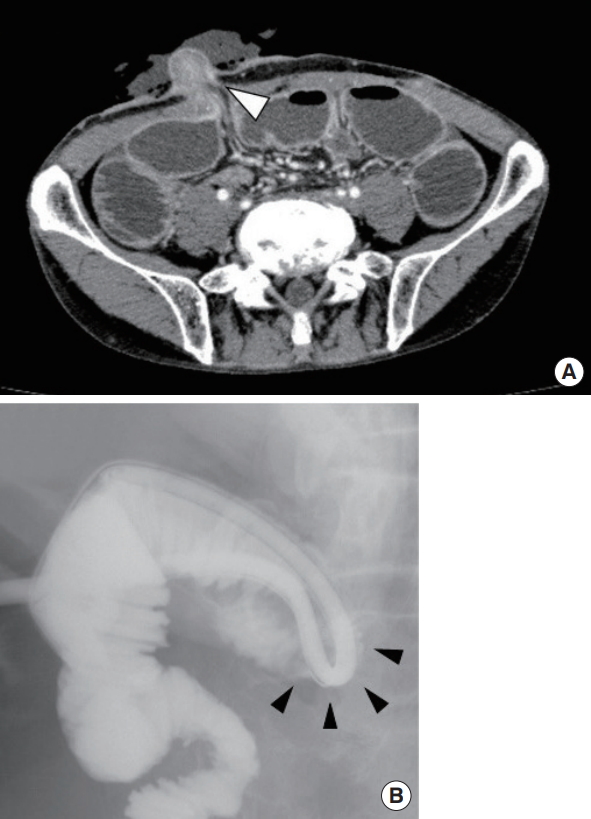
Diagnosis of small bowel obstruction based on radiographic findings. (A) Computed tomography scan examination shows small intestine stenosis at the level of the abdominal wall (arrowhead) and dilatation of the oral side of the small intestine. (B) Contrast radiography from the transstomal decompression tube shows intestinal pinching at the oral side of the loop ileostomy (arrowheads).
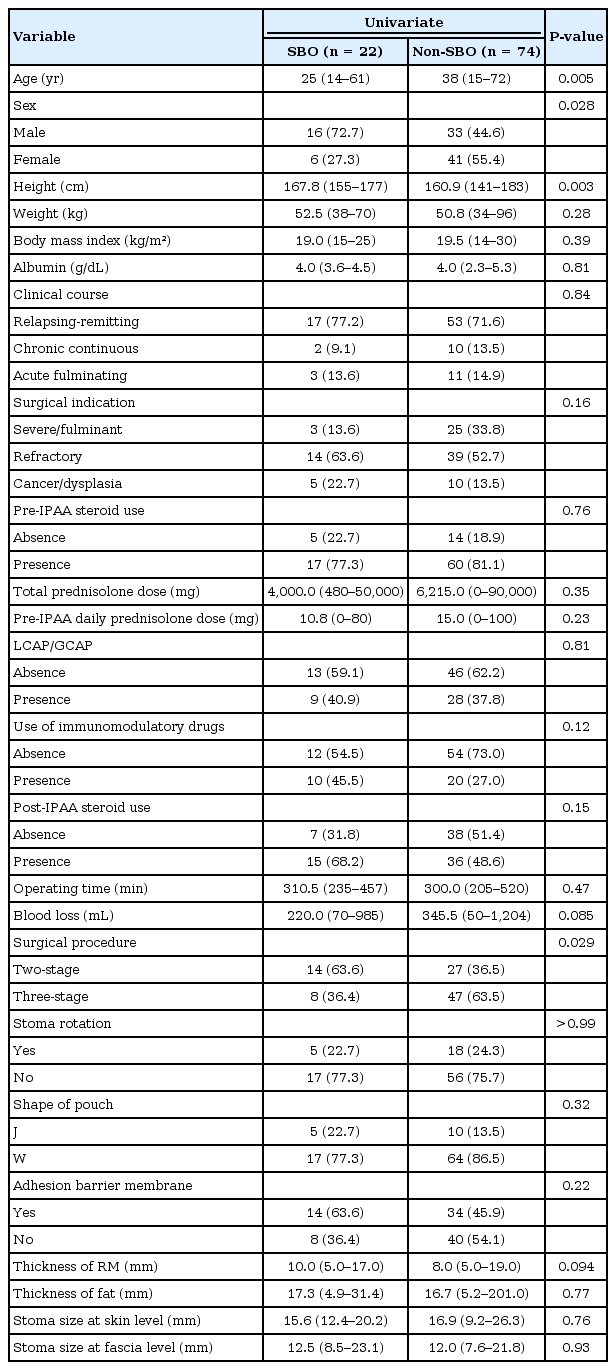
According to the univariate analysis, younger age (P = 0.005), male sex (P = 0.028), taller height (P = 0.003), and a 2-stage procedure (P = 0.029) were risk factors associated with a SBO after an IPAA with a loop ileostomy in patients with UC (Table 2). No differences were observed in the BMI and the preoperative serum albumin level as parameters used to evaluate the nutritional state. The median thickness of the abdominal rectus muscle was 10.0 mm in the SBO group and 8.0 mm in the non-SBO group. In addition, the thickness of the subcutaneous fat was 17.3 mm in the SBO group and 16.7 mm in the non-SBO group. These parameters on the CT scan image were not significantly different between the 2 groups. The parameters of stoma size at the skin or the fascia level on the CT scan were also not significantly different. In the multivariate logistic regression analysis, the 2-stage procedure (odds ratio, 2.850; 95% confidence interval, 1.009–8.044; P = 0.048) was a significant independent risk factor of a SBO after an IPAA with a loop ileostomy in patients with UC (Table 3).
DISCUSSION
A SBO remains a common complication after pelvic or abdominal surgery. A previous report noted that a SBO after abdominal surgery occurred in approximately 20% of the patients [1]. The risk of a SBO is greater, in particular, after colorectal surgery [21]. The TPC with an IPAA has become the standard surgical approach for treating patients with UC. The incidence of a SBO after an IPAA for UC is higher than that observed with other types of abdominal surgery. As the IPAA may be considered a high-risk procedure for developing a SBO, it can be combined with a wide abdominal and pelvic dissection and may be performed as a staged surgical procedure. Moreover, a diverting loop ileostomy is associated with an increased risk of a SBO [7, 16].
In the present study, the incidence of a SBO in the pretakedown of a loop ileostomy after an IPAA was 22.9% (22 patients). Furthermore, of these 22 patients, 11 required surgical intervention. We had to close the ileostomy earlier than expected. The cause of surgical intervention in 8 of the 11 patients was an outlet obstruction at the level of the abdominal wall. In general, surgical treatment for a SBO should be considered when the patient’s symptoms do not resolve within 6 days of nasogastric decompression [21, 22]. The incidence of a SBO is not rare after an IPAA with a loop ileostomy for patients with UC. If the SBO does not improve after conservative treatment, due consideration should be given to an earlier closure of the ileostomy.
In our study, we also evaluated the relationship between the parameters on the CT scan image and the incidence of a SBO after an IPAA in patients with UC. We measured some parameters on the CT scan image, such as the thickness of the abdominal rectus muscle, the thickness of the subcutaneous fat, and the size of the stoma at the skin or fascia level. Even though no statistically significant differences in these parameters from the CT scan image with respect to the incidence of a SBO after an IPAA with a loop ileostomy were seen in our study, the thickness of the abdominal rectum muscle may be a risk factor of a SBO (P = 0.094).
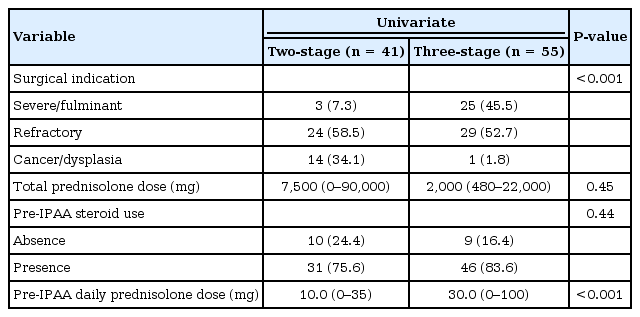
The current study showed that a 2-stage procedure is an independent risk factor for a SBO after an IPAA with a loop ileostomy in patients with UC. MacLean et al. [11] also reported that the rate of a SBO occurring in patients who had their colectomy performed before the IPAA procedure was lower. Our study corroborated these results. The reason for the difference in the incidence of a SBO between the 2- and the 3-step procedures may be partly due to the steroid dose. A previous report noted that the incidence of a SBO in the 3-stage procedure was lower because a high dose of steroids may reduce the risk of adhesions [11]. In our study, the median total dose of prednisolone before the IPAA was 7,500 mg for patients undergoing the 2-stage procedure and 2,000 mg for the patients undergoing the 3-stage procedure (P = 0.45). However, the pre-IPAA daily prednisolone dose for the patients undergoing the 3-stage procedure was higher than that of the patients undergoing the 2-stage procedure (Table 4). No difference in the use of perioperative steroids was observed between the SBO and the non-SBO groups (Table 2). Therefore, further investigation is required to understand the effect of a difference in steroid use on the incidence of a SBO.
Recently, laparoscopic surgery has been more frequently used for UC than open surgery has [23-26]. The laparoscopic TPC with an IPAA is feasible and may provide better short-term advantages, including a shorter duration of hospital stay and a lower rate of complications [24]. A laparoscopic approach may result in decreased adhesion formation and a lower risk of a subsequent SBO [11]. However, Dolejs et al. [25] stated that the incidences of a SBO were 7% after open surgery and 13% after laparoscopic surgery in patients with UC. Furthermore, El-Gazzaz et al. [26] noted that the incidences of a SBO after open and laparoscopic surgeries were 4% and 6%, respectively. These authors considered the intestine to be potentially more prone to mechanical kinking or twisting at the ileostomy site because fewer adhesions were generated during laparoscopic surgery. However, they also stated that the laparoscopic group may have a lower incidence of SBO over a longer period.
A diverting loop ileostomy is also a risk factor for a SBO. In this study, the cause of the SBO in eight patients was considered to be an outlet obstruction. Of these patients, 6 had undergone a 2-stage procedure. We consider the loop ileostomy to be one of the main causes of an outlet obstruction. In general, the procedure of the IPAA without a diverting ileostomy may increase anastomotic leakage [27]. However, an IPAA without a protective ileostomy is a safe option in the pediatric population and is not associated with increased morbidity [6]. Therefore, the omission of a covering ileostomy may still be justified in patients defined as low risk.
As ways to prevent a SBO after surgery, several clinical studies reported that employment of a sodium hyaluronate-based bioresorbable membrane (Seprafilm; Genzyme Corp., Cambridge, MA, USA) markedly reduced the incidence and the severity of adhesions after abdominal surgery, although we did not observe any differences in adhesion barrier use in our study [28]. The intraluminal pressure of the small intestine is estimated to be 6–13 mmHg and that of the colon to be 10–30 mmHg [29, 30]. Considering that the intraluminal pressure of the small intestine is lower than that of the colon, a possibility exists that relative stenosis in the abdominal wall level may have occurred [29]. Consequently, making an abdominal wall opening of sufficient size to prevent a “pinching” effect and edema is important [12]. However, determining an appropriate stoma size in this study was difficult.
The rotation of the loop ileostomy is believed to facilitate emptying into the appliance and to prevent spillage into the efferent limb protecting the anastomosis. However, rotated and nonrotated loop ileostomies are equally effective in division. Indeed, rotation of the loop ileostomy is a risk factor for intestinal obstruction. The incidence of obstruction in patients with rotated ileostomies was nearly twice that in patients with nonrotated iloestomies [16]. We consider that stoma rotation may induce twisting of the small intestine. Therefore, we have not performed a recent rotation of the loop ileostomy.
Our study has several limitations. It was a retrospective study with a small sample size. Furthermore, the backgrounds of the patients who underwent the 2- and the 3-stage procedures were different (Table 4). The differences in the variables between the 2 groups may be due to the selection bias when performing the operation because we selected the 3-stage procedure for patients with severe conditions, like severe/fulminant type UC. In addition, a SBO in this study included an outlet obstruction and adhesive ileus as the reason for the obstruction. In this study, the cause of the SBOs requiring surgery was “outlet obstruction” in 8 patients. In the remaining three patients, the cause of the SBO was considered to be intra-abdominal adhesion. However, clearly distinguishing between these 2 causes is difficult. Nevertheless, our study has demonstrated new and significant findings related to the risk factors associated with the ileostomy pretakedown period after an IPAA in patients with UC. In conclusion, the present study revealed that the 2-stage procedure is a significant and independent risk factor of a SBO occurring after an IPAA with a loop ileostomy in patients with UC.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.