Risk factors associated with low anterior resection syndrome: a cross-sectional study
Article information
Abstract
Purpose
Oncological outcomes following rectal cancer surgery have improved significantly over recent decades with lower recurrences and longer overall survival. However, many of the patients experienced low anterior resection syndrome (LARS). This study identified the prevalence and risk factors associated with the development of LARS.
Methods
This cross-sectional study involved patients who were diagnosed with rectal cancer and had undergone sphincter-preserving low anterior resection from January 2011 to December 2020. Upon clinic follow-up, patients were asked to complete an interviewed based questionnaire (LARS score) designed to assess bowel dysfunction after rectal cancer surgery.
Results
Out of 76 patients, 25 patients (32.9%) had major LARS, 10 patients (13.2%) had minor LARS, and 41 patients (53.9%) had no LARS. The height of tumor from anal verge showed an association with the development of major LARS (P=0.039). Those patients with less than 8 cm tumor from anal verge had an increased risk of LARS by 3 times compared to those with 8 cm and above (adjusted odds ratio, 3.11; 95% confidence interval, 1.06–9.13).
Conclusion
Results from our study show that low tumor height was a significant risk factor that has a negative impact on bowel function after surgery. The high prevalence of LARS emphasizes the need for study regarding risk factors and the importance of understanding the pathophysiology of LARS, in order for us to improve patient bowel function and quality of life after rectal cancer surgery.
INTRODUCTION
Colorectal cancer (CRC) is one of the leading cancers worldwide as well as in Malaysia. Malaysia was the third highest overall CRC incidence (18.30 per 100,000) in South East Asia, after Singapore and Brunei [1]. Malaysia reported an 11.3% increase in new cancer cases from 103,507 in 2007–2011 to 115,238 in 2012–2016. It was the most common cancer in men and second in women in Malaysia according to Malaysian National Cancer Registry report 2012–2016. Chinese ethnicity has the highest incidence, followed by Malay and Indian [2]. Left-sided carcinoma is the commonest form and constitutes 81.8% of all notified cases [3].
Historically, low-lying rectal cancer was treated with abdominal perineal resection, which was considered the gold standard. During the past decades, the approach and treatment of rectal cancer have improved and advanced markedly with the introduction of neoadjuvant therapy and innovation and advent of better surgical techniques and equipment. Low anterior resection (LAR) and total mesorectal excision (TME) with or without diversion stoma has become the preferred surgical procedure in a patient with resectable rectal cancer, with the intention to preserve anal sphincter and avoid permanent stoma [4].
Oncological outcomes following rectal cancer surgery have improved significantly over recent decades with lower recurrences and longer over survival. However, these survival advantages have greatly overshadowed functional outcomes of surgery, which are poor for many patients and consistently under-reported [5]. Anatomical preservation in sphincter preserving surgery does not always mean a perfect restoration of anorectal function. Many of the patients experienced several bowel symptoms after surgery, which include flatus and fecal incontinence, frequent bowel opening, urgency, or sense of incomplete defecation. This combination of such symptoms after sphincter preserving surgery is referred to as LAR syndrome (LARS) and it can be associated with significant negative impact on quality of life [6].
A pragmatic definition of anterior resection syndrome is disordered bowel function after rectal resection, leading to detriment in quality of life. These symptoms usually improved a few months after surgery, and reach plateau within 2 years. Studies have shown the presence of adverse symptoms after surgery can be long term and up to 15 years after surgery, with the prevalence of obstructed defecation symptoms from 12% to 73% and fecal incontinence symptoms varying from 0% to 71% [5]. The term "low anterior resection syndrome" is more commonly used due to its association with lower resection and anastomosis [7].
LARS has a major impact on the quality of life depending on the severity of the symptoms. Many patients suffer from disability, describing social limitations, and developing psychiatric disorders [8]. The estimated prevalence of LARS was reported to be ranging from 19% to 52% in variety of studies [9]. The use of different assessment and collection tools that are not specific to LARS leads to such discrepancies in the data collection for prevalence. A validated scoring system specific to LAR was introduced by Emmertsen and Laurberg [10], taking into consideration of bowel dysfunction and its overall impact on patient quality of life. This allows the collection of comparable data as well as makes meta-analysis possible.
Despite LARS being a significant and rising concern among surgeons and patients, the exact pathophysiology was not fully understood. Recent studies have addressed possible factors contributing to LARS, such as age, surgical technique (total or partial mesorectal excision), type of anastomosis, adjuvant therapy, neoadjuvant therapy, and postoperative complications (anastomosis leakage or stricture) [11].
Since patients who undergo a sphincter-sparing rectal resection are at risk of developing LARS, perioperative efforts should be made to prevent LARS. The aim of this study was to identify the prevalence of LARS among patients who underwent sphincter preserving rectal surgery and clinical risk factors associated with the development of LARS in our populations.
METHODS
Ethics statement
The study was approved by the Human Research Ethics Committee of Universiti Sains Malaysia (No. USM/JEPeM/19120879) and by the Medical Research and Ethics Committee of the Ministry of Health Malaysia (No. NMRR-19-3385-51510 (IIR)). All participants have provided written informed consent.
Study design and setting
This is a cross-sectional study of patients with rectal cancer who had undergone sphincter-preserving anterior resection at Hospital Raja Perempuan Zainab II (HRPZ II) in Kota Bharu, Malaysia and Hospital Universiti Sains Malaysia (HUSM) in Kubang Kerian, Malaysia. These 2 hospitals are the main and referral hospitals with colorectal units in Kelantan, Malaysia.
Participants
All patients who were diagnosed with rectal cancer and had undergone sphincter-preserving anterior resection (anterior resection, LAR, and ultra-LAR) at the participating hospitals between January 2011 and December 2020 were recruited. All patients with age more than 18 years old and above, and had restored bowel continuity for at least 12 weeks were included while patients with stoma, recurrent disease, and intellectual disabilities were excluded.
The sample size for this study was calculated using the PS: Power and Sample Size Calculation ver. 3.0.12 (DalePlummer) with the significance level (α) of 0.05, and the power of study (1–β) of 80% based on the parameter estimate obtained from Jimenez-Gomez et al. [12]. The final targeted sample size was determined by considering 10% dropout rate. The estimated sample size for this study was 84 samples.
Data collection
The data of these patients were retrieved from the record units and operation theatres at HRPZ II and HUSM. Upon entry into the study, upon clinic follow-up, patients were asked to complete an interviewed based questionnaire designed to assess bowel dysfunction after rectal cancer surgery in a colorectal clinic. The rest of clinical variables were retrieved from patient case notes and electronic records.
All the patients were interviewed only by the principal investigator to reduce the risk of bias. Clinical data from the questionnaires were collected and inserted into a LARS database. Patients were grouped into 2 separate cohorts (those with major LARS scores and those with minor or LARS symptoms). Categorical outcomes were compared for the major LARS group.
A proforma checklist was used to guide data extraction and the following data were recorded from patient case notes: demographic data (age at surgery, sex, and race); type of surgery (emergency or elective); approach (either laparotomy or laparoscopic or laparoscopic converted to laparotomy); type of resection (anterior resection, LAR, or ultra-LAR); tumor demographic (staging, height of tumor from anal verge, and location of tumor in rectum); neoadjuvant therapy (short- or long-course concurrent chemoradiation or any adjuvant therapy given postoperatively); any creation of diversion stoma and duration of patient on diversion stoma; and postoperative complications (anastomotic leak, abscess, ileus, and anastomotic stricture).
LARS score
The research tool used in this study is an international validated scoring system, the LARS score [10, 13]. The LARS score is a simple 5-questions tool that was first created in 2012 in Denmark and has been validated in English translation in 2015 by Juul et al. [14]. Those with a LARS score of 30 to 42 were regarded as having major LARS while those scoring 21 to 29 and 0 to 20 were categorized as having minor LARS and no LARS, respectively. The questionnaire used in this study was obtained with permission. On the European Society of Coloproctology (ESCP) website, LARS scoring instructions are available together with the Bowel Function Questionnaire in 24 languages and Malay language was one of the translated versions.
Data analysis
Descriptive statistics were used to summarize the sociodemographic characteristics of subjects. Numerical data were presented as means±standard deviations (SDs) or medians (interquartile ranges) based on their normality distribution. Categorical data were presented as frequency (percentage). Each specific objective was analyzed descriptively. The data were analyzed by univariable analysis (simple logistic regression) and multivariable analysis (multiple logistic regression).
The independent variable involved in this study were numerical category (age and duration on diversion stoma); categorical group (operation, staging, T category, N category, height of tumor from anal verge, location of tumor in rectum, neoadjuvant therapy, formation of stoma, and any postoperative complication such as leaking, abscess, ileus, or stricture). In the simple logistics regression analyses, one by one variable was tested and variables with a P-value of < 0.25 were included in the next analysis (multiple logistics regression). Variables with a P-value of <0.25 were age at surgery, the height of tumor from anal verge, stoma, duration on diversion stoma, and abscess. Backward and forward logistic regression methods were applied in the multiple logistics regression in selecting the most influenced variables associated with the development of LARS among rectum cancer patients. All odds ratios (ORs) were presented with 95% confidence intervals (CI). Statistical analysis was carried out using IBM SPSS ver. 22.0 (IBM Corp). The limit of significance was set at 0.05.
RESULTS
Patient demographics and clinical characteristics
The calculated sample size including a 10.0% dropout rate was 84. However, only 76 data of the patients were available during the data collection period, notably due to low patient turn-up rates in the clinic during the COVID-19 period. These 76 patients who fulfilled subject criteria recruited from those who had sphincter preserving rectal surgery for rectal cancer performed in HRPZ II and HUSM from January 2011 until December 2020 had achieved the optimum sample required with the power of study of 80.0%.
Forty-two patients (55.3%) were male. Majority of the patients (n=71, 93.4%) were Malay. This is consistent with demographic data of Kelantan, Malaysia population where more than 90% of the populations are Malay. The mean age of patients when they had surgery was 60.9 years, and 56.6% of patients were 60 years and above when surgery was performed (Supplementary Table 1).
The majority of surgery (94.7%) was performed as elective procedures as most of the patients presented with obstructed tumor had trephine diversion stoma prior to definitive surgery, rather than single-staged surgery. Eighty percent of the surgery was done with laparotomy approach due to tumor factor as well as surgeon preferences. According to LARS score, there were 25 patients (32.9%) with major LARS, 10 patients (13.2%) with minor LARS, and 41 patients (53.9%) with no LARS. The mean±SD of duration from surgery performed until the questionnaire is 34.8±21.37 months as patient was collected over a period of 10 years (Supplementary Table 2).
For the tumor demographic, 19 patients (25.0%) presented with low rectum tumor where tumor height was less than 8 cm from anal verge, and among this group of patients, 40% of them experience major LARS (Supplementary Table 3).
Twenty-three patients (30.3%) with rectal cancer in our study received neoadjuvant concurrent chemoradiotherapy (CCRT) prior to surgery and 37 patients (48.7%) received adjuvant therapy either chemotherapy or both chemotherapy with radiotherapy (Supplementary Table 4). There was no case of radiotherapy alone in the neoadjuvant or adjuvant setting. Only long-course neoadjuvant CCRT was given to our patient.
The stoma is created for the purpose of either preoperatively obstructed tumor prior to definitive surgery or as a diversion intraoperatively for low anastomosis. Fifty-seven patients (75.0%) had a stoma created and 36.8% of them had major LARS after reversal of the stoma. The mean duration of patients on stoma prior to reversal was 13 months where long mean duration was noted in patients who developed LARS (Supplementary Table 5).
Factors associated with the development of LARS among rectum cancer patients
Table 1 shows univariable analyses on the factors associated with the development of LARS among rectum cancer patients. Using simple logistic regression, variables with a P-value less than 0.25 were age at surgery, height of tumor from anal verge, stoma, duration on diversion stoma, and abscess.
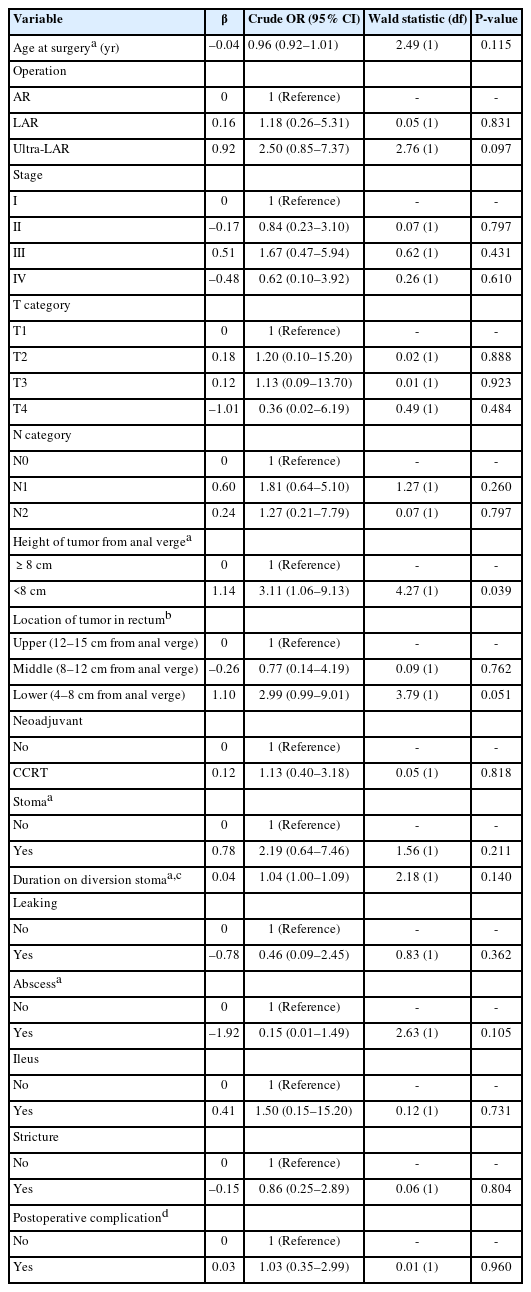
Factors associated with the development of LARS among rectum cancer patients from simple logistics regression analyses (n=76)
Multiple logistic regression showed that the height of tumor from anal verge was the factor associated with the development of LARS among rectum cancer patients. After adjustment for age at surgery, the height of tumor from anal verge, stoma, duration on diversion stoma, and abscess, the results showed that patients with less than 8 cm tumor from anal verge had an increased risk of LARS by 3 times compared to patients with 8 cm and above (adjusted OR, 3.11; 95% CI, 1.06–9.13; P=0.039).
DISCUSSION
With the advancement of neoadjuvant therapy and surgical technique, overall survival for rectal cancer has increased compared to previously. Apart from the oncological outcome, more attention is being paid to functional outcomes in patients who underwent sphincter preserving rectal cancer surgery. Severe bowel dysfunction has led to a negative impact on patients’ quality of life and affects their day-to-day activities. Thus, it is important to evaluate bowel symptoms during follow-up and risk factors associated with the development of LARS need to be studied. Studies have shown that functional disturbances are most prominent during the first 12 months before stabilizing into long-term dysfunction [6]. Evidence has also shown that LARS could be permanent changes rather than short-term rectal irritability as adverse effects still present after 15 years following surgery [15].
A meta-analysis conducted in 2018 [16] has shown bowel dysfunction assessment after surgery was inconsistent because the majority of the studies have used nonvalidated questionnaires. The introduction of LARS score allows the collection of comparable data and studies with higher-level evidence such as meta-analyses can be conducted. Our study showed a prevalence of major LARS of 32.8%, which is consistent with the majority of the studies. The meta-analysis by Croese et al. [16] reported prevalence ranged from 17.8% to 56%, and all primary studies included had assessed prevalence of LARS using LARS score and thus offered a more accurate representative of the prevalence.
LARS has gained more attention in recent years and substantial research was conducted to study the cause of LARS. However, the definite cause and pathophysiology for the development of LARS and its severity is not clearly understood yet. It is believed that symptoms of LARS are usually caused by multifactorial which include impaired anal sphincter function, neorectal reservoir dysfunction, and colonic dysmotility. An impaired anal sphincter can be the result from rectal mobilization and mesorectum dissection, mechanical tear during stapler insertion [17] or the effect of pelvic radiotherapy, and these can be manifested as urgency and incontinence [5]. Neorectal reservoir dysfunction can be the result of denervation during surgery dissection and pelvic radiotherapy leading to hyposensitivity due to impaired afferent nerve function [18]. It can also be due to reduced rectum capacity and compliance after surgery, thus smaller amount of feces is sufficient to stimulate defecation reflex causing stool frequency, urgency, frequent and incontinence. However, construction of neorectal reservoir such as colonic J-pouch only provide symptomatic improvement of less than 18 months, but no long-term benefit [19].
Various factors have been reported to be possibly associated with LARS in multiple studies conducted past decade, which include female sex, tumor height, level of anastomosis, neoadjuvant or adjuvant radiation therapy, presence of stoma, and postoperative complications. In our study, we found no significant differences in the development of LARS between sex and different age group. Only 1 study [20] reported that age of >70 years was associated with LARS (P=0.003), but the results were not significant in other studies.
This study investigated the possible factors that predispose to the development of major LARS using an international validated LARS score in 76 patients. Our results suggested that tumor height of less than 8 cm from anal verge was a significant factor in the development of major LARS (P=0.039). Tumor height and anastomosis height was one of the statistically significant variables affecting major LARS which was reported in a few other studies [10, 20, 21]. Lower tumor height will result in less rectum remnants after surgery in order to achieve oncological resection margin. A low rectal tumor will invariably require TME during resection of primary tumor. TME was also reported to be significant factor associated with the development of LARS in a study by Carrillo et al. [22]. Although height of the tumor and anastomosis height was not discussed in that particular study, TME was performed for middle and low rectal tumor.
The rectum functions as a reservoir for stools, more rectum has to be resected in the low-lying tumor, thus leaving behind a smaller neorectal reservoir. Conventional end-to-end colorectal or coloanal anastomosis was believed to contribute to symptoms of LARS such as incontinence and urgency. Various alternative configuration techniques have been introduced, these include side to end anastomosis, colonic pouch, or transverse coloplasty. Early data suggested that patients who received reconstructive neorectal reservoir had a better functional bowel outcome in 12 months compared to end-to-end anastomosis. However, the frequency in the end-to-end anastomosis group reduced to that of pouch group by 24 months after surgery [23, 24]. The effect of neorectal volume in relation to the functional outcome remain unclear and offer no long-term benefits in any of the reconstructive technique [19].
Among all, neoadjuvant or adjuvant radiotherapy was one of the most consistent factors assessed and has shown to be statistically significant in the majority of the studies. Radiotherapy plays a critical role in the treatment of rectal cancer either as neoadjuvant or adjuvant therapy in adjunct to surgical resection. Despite having better local recurrence outcome and increase overall survival [25], pelvic radiation is not without risk. Multiple studies including a meta-analysis have consistently reported neoadjuvant radiotherapy as an independent risk factor for the development of LARS [9, 16, 21, 26, 27]. This is probably related to pelvic floor nerve damage and neorectal hyposensitivity as a complication from pelvic radiation. However, our study failed to show a relationship between radiation and poor bowel function. This is probably due to some patients in our study refusing radiotherapy as part of their treatment, and the lack of radiotherapy services in one of our centers might contribute as well.
There is conflicting data on diversion stoma and duration of stoma in relation to poorer bowel function after anterior resection. Our study has not shown any association between the stoma and major LARS. A recent randomized controlled trial in 2017 [28] compared LARS score in patient treated with diversion stoma and without a stoma, had found no statistically different in major LARS when comparing both groups. However, few other studies have shown that the presence of diversion stoma is statistically associated with major LARS [9, 22]. Only 1 study [27] reported that stoma closure after 1 year had increased risk of major LARS. The association was believed due to confounding factor rather than diversion stoma itself. Usually diversion stoma is created for low-lying tumor with lower anastomosis, which is again combined with TME, which both are known to increase risk of LARS.
Common complications occur after anterior resection include anastomotic leak, pelvic abscess, ileus, and anastomotic stricture. Some studies have shown an increased risk of major LARS following anastomotic leak; however, the results were not consistent. The anastomotic leak can lead to adverse bowel symptoms manifested as increase frequency and incontinence, as well as impaired quality of life [29]. In the study, anastomotic leak and other complications like pelvic abscess, ileus, and anastomotic stricture were not associated with major LARS. The study also tried to evaluate the association between the approach of surgery and the development of major LARS. In our study, both laparotomy and laparoscopic approaches have not shown any statistically significant. This result is expected as we believe careful dissection during the surgery rather than the approach is more important to prevent denervation of pelvis nerve plexus.
There were certain limitations within this current study. Firstly, the questionnaire was delivered only to those patients who follow up in the colorectal clinic within the study period. However, we managed to get 76 patients to answer the questionnaire despite limited clinic consultation and a low turn-up rate of patients where the healthcare is burdened with COVID-19 during the study period. In order to achieve a large cohort of patients to study the association between various risk factors and LARS, retrospective data were collected from 2011 until 2019. Thus, the patient who underwent surgery earlier will have a longer duration before they answer the questionnaire. This might lead to an underestimation of the prevalence of LARS especially in the patient who had surgery earlier. The nature of anastomosis and chemotherapy agents was not assessed in this study due to their heterogenicity.
In conclusion, using an internationally validated questionnaire LARS score, our study reports that 1/3 of patients (32.8%) developed major LARS following sphincter preserving surgery for rectal cancer. This study identified low tumor height as a significant risk factor that has a negative impact on bowel function after surgery. The high prevalence of LARS emphasizes the need for study regarding risk factors and the importance of understanding the pathophysiology of LARS, in order for us to improve patient bowel function and quality of life after rectal cancer surgery.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: WZWZ, ASMS; Data curation: SLL, WZWZ; Formal analysis: all authors; Supervision: WZWZ, ASMS; Writing–original draft: Z Zahari, WZWZ; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Supplementary materials
Supplementary Table 1.
Demographics of rectal cancer patients
Supplementary Table 2.
Surgery descriptions of rectal cancer patients
Supplementary Table 3.
Tumor descriptions of rectal cancer patients
Supplementary Table 4.
Neoadjuvant and adjuvant treatment for rectal cancer patients
Supplementary Table 5.
Diversion stoma among rectal cancer patients
Supplementary materials are available from https://doi.org/10.3393/ac.2022.00227.0032.

