Partial mesorectal excision can be a primary option for middle rectal cancer: a propensity-score matched retrospective analysis
Article information
Abstract
Purpose
Although partial and total mesorectal excision (PME and TME) is primarily indicated for the upper and lower rectal cancer, respectively, few studies have evaluated whether PME or TME is more optimal for middle rectal cancer.
Methods
This study included 671 patients with middle and upper rectal cancer who underwent robot-assisted PME or TME. The 2 groups were optimized by propensity-score matching of sex, age, clinical stage, tumor location, and neoadjuvant treatment.
Results
Complete mesorectal excision was achieved in 617 of 671 patients (92.0%), without showing a difference between the PME and TME groups. Local (5.3% vs. 4.3%, P>0.999) and systemic (8.5% vs. 16.0%, P=0.181) recurrence rates also did not differ between the 2 groups, respectively, in patients with middle and upper rectal cancer. The 5-year disease-free survival (81.4% vs. 74.0%, P=0.537) and overall survival (88.0% vs. 81.1%, P=0.847) rates also did not differ between the PME and TME groups, confined to middle rectal cancer. Moreover, 5-year recurrence and survival rates were not affected by distal resection margins of 2 cm (P=0.112) to 4 cm (P>0.999), regardless of pathological stages. Postoperative complication rate was higher in the TME than in the PME group (21.4% vs. 14.5%, P=0.027). Incontinence was independently associated with TME (odds ratio [OR], 2.009; 95% confidence interval, 1.015–3.975; P=0.045), along with older age (OR, 4.366, P<0.001) and prolonged operation time (OR, 2.196; P=0.500).
Conclusion
PME can be primarily recommended for patients with middle rectal cancer with lower margin of >5 cm from the anal verge.
INTRODUCTION
Miles’ groundbreaking approach to abdominoperineal resection enlightened the first concept of mesorectal excision in 1908 [1]. The mesorectum was described as a clue to pelvic recurrence of rectal cancer, defining the principle of total mesorectal excision (TME) by Heald et al. [2]. Complete removal of the mesorectal fascia, the sites of potential tumor spread, in 50 patients with Dukes B/C rectal cancer who underwent TME resulted in a 2-year local recurrence rate of 0%. A local recurrence rate of ≤ 10% is generally expected if proper TME techniques are employed [3].
The completeness of mesorectal excision (complete mesorectal excision) is of prime importance, as resection at the muscularis propria plane results in ≥ 2-fold higher risks of both local and systemic recurrences than resection at the (intra)mesorectal plane [4]. However, routine TME along all levels of rectal cancer may be suboptimal, due to increased risks of morbidity, including ≥ 10% higher rates of anastomotic leakage and lower anterior resection syndrome than partial mesorectal excision (PME) [5-7]. Few studies have compared outcomes of PME and TME as a function of tumor location, other than narrative reviews suggesting that PME is optimal for upper rectal and rectosigmoid cancers, whereas TME is optimal for middle and lower rectal cancers [3, 5]. TME, however, may be confused with PME when dissection is performed down to the mesorectal end without complete excision of the mesorectum [8].
Furthermore, as TME included complete excision of mesorectal fascia, regardless of extent, TME indispensably excised adjacent tissues via en bloc excision including aggressive tumor deposits (ATDs) or suspicious invasion. Therefore, TME-associated histopathological changes correlate with R0 resection and consequent anorectal dysfunction needs to be reconsidered. Otherwise, incomplete lateral pelvic mesorectal excision was associated with increased local recurrence, indicating that complete lateral pelvic mesorectal excision is an indispensable component of quality TME in a previous study [9].
The current study was designed to compare prognostic and functional outcomes in propensity-score matched patients with middle and upper rectal cancer who underwent PME and TME. These findings further suggest the mandatory extent of mesorectal excision for middle rectal cancer.
METHODS
Patients and study design
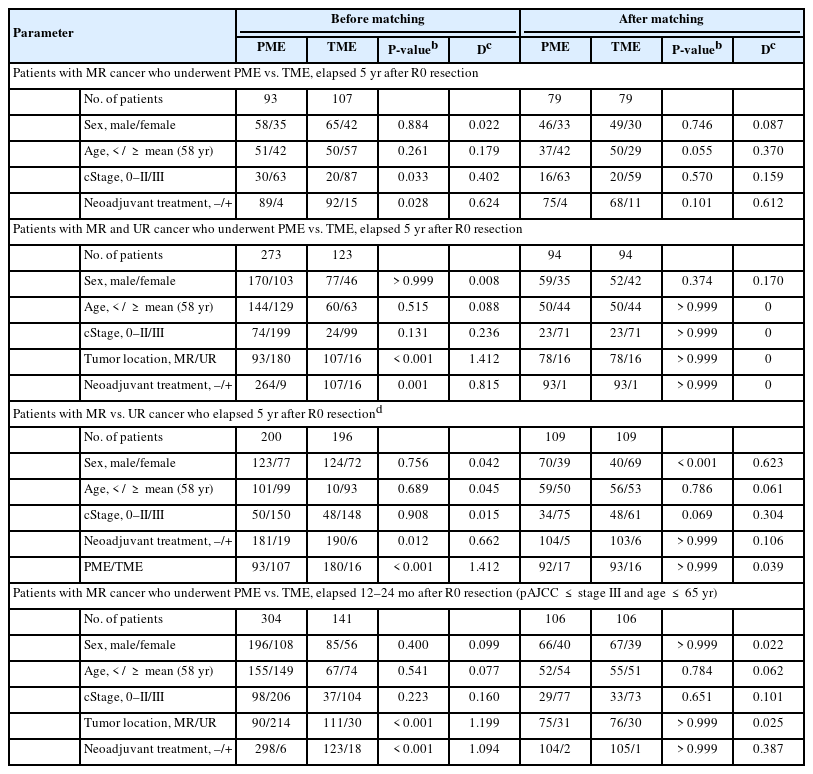
The medical records of the 1,402 consecutive patients who underwent robot-assisted curative-intent lower anterior resection (LAR) from 2010 to 2021 at Asan Medical Center (Seoul, Korea) were reviewed. Of these patients, 671 (47.9%) underwent LAR for middle and upper rectal cancers, comprising 437 and 234 underwent PME and TME, respectively. Patients were subsequently subjected to 1:1 propensity-score matching by sex, age, clinical stage, tumor location, and neoadjuvant treatment (Table 1). Tumor location was divided into the lower (≤ 5 cm from the anal verge), middle (> 5 and ≤ 10 cm), and upper (> 10 and ≤ 15 cm) rectum [10]. The primary end point was mesorectal quality in the PME and TME groups. Secondary end points were anorectal function 12 to 24 months after surgery, and 5-year cumulative recurrence and disease-free/overall survival (DFS/OS) rates in patients aged ≤ 80 years with clinical American Joint Committee on Cancer (AJCC, 8th ed; https://www.cancer.org/) stage ≤ III disease. A detailed study scheme, with inclusion and exclusion criteria, is illustrated in Fig. 1. All patients provided voluntary written informed consent, and the study protocol, conforming the Declaration of Helsinki, was approved by the Institutional Review Board of Asan Medical Center (No. 2022-0683).
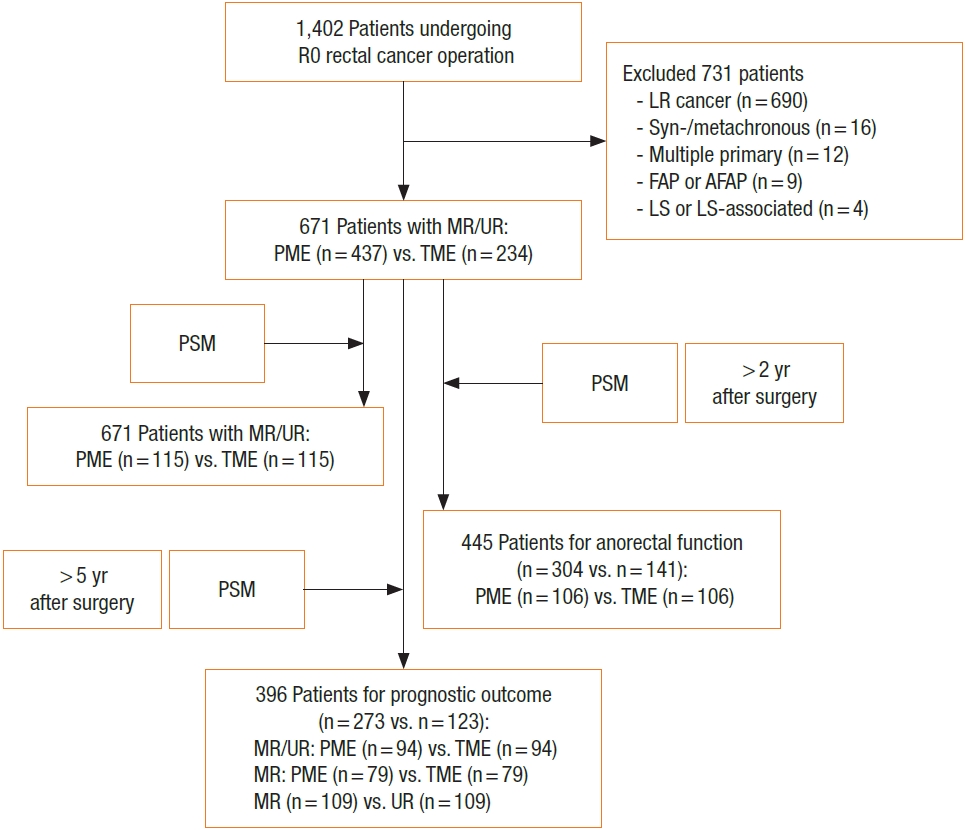
Flow diagram of the study protocol. R0, curative resection with microscopically margin-negative resection; LR, lower rectum (≤5 cm from the anal verge); FAP, familial adenomatous polyposis; AFAP, attenuated FAP; LS, Lynch syndrome; MR, middle rectum (>5 to 10 cm from the anal verge); UR, upper rectum (>10 to 15 cm from the anal verge); PME, partial mesorectal excision; TME, total mesorectal excision; PSM, propensity-score matching; postop, postoperative.
Operation and adjuvant treatment
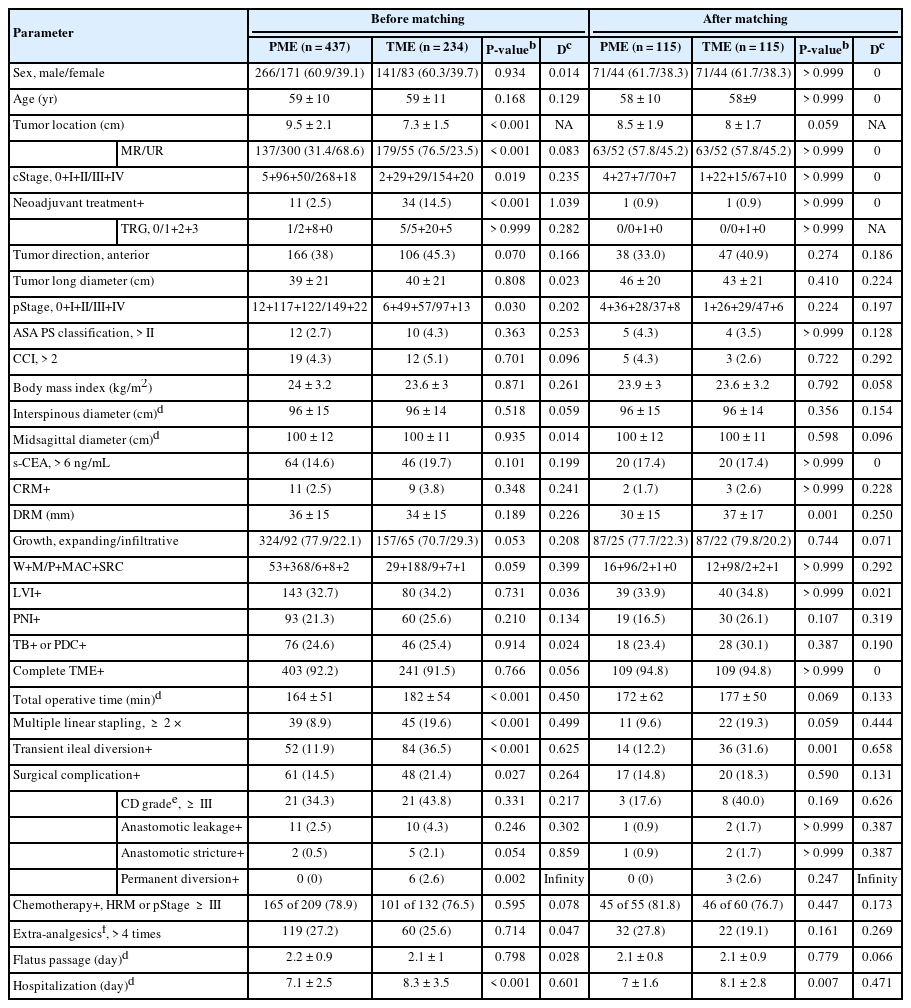
Neoadjuvant treatment was recommended for patients with clinical stage III or T4 middle rectal cancer, with most of the tumor located below the peritoneal reflection. In contrast, upfront surgery was also considered for patients with cT3a/bN1–2, classified as low to intermediate risk based on the European Society for Medical Oncology guidelines [10, 11]. Final treatment modality was determined after informed discussions between patients and surgeons, with recommendations by the multidisciplinary team. Neoadjuvant therapy and postoperative chemoradiotherapy for middle rectal cancer, consisting of long-course radiotherapy with boost chemotherapy, were as described [11]. Patients with pathologic AJCC stage (pStage) ≥ III or high-risk stage II cancer [10] were administered adjuvant chemotherapy, consisting primarily of 5-fluorouracil/leucovorin or capecitabine, but sometimes oxaliplatin (Table 1).
Operative procedure and quality assessment
TME extent was determined according to tumor location and distal resection margin (DRM). Briefly, the inferior mesenteric artery (IMA) was ligated and excised immediately after left colic artery bifurcation (low ligation), followed by downward dissection, alternating between the right and left sides in a spiral manner. Particular attention was needed for mesorectal excision posterior to Denonvilliers fascia, and laterally at the fusion of the proper rectal fascia and prehypogastric fascia wrapping the inferior hypogastric nerves, preserving the pelvic autonomic nerves (Fig. 2). TME was completed at the end of the mesorectal fascia just above the anorectal canal. By contrast, PME consisted of mesorectal excision transverse to the rectum depending on the DRM, which was conventionally defined as 2 to 4 cm for middle and upper rectal cancers [12]. Completeness of mesorectal excision was assessed by examining mesorectal quality, including bulk, defects, coning, and circumferential resection margin (CRM) [4]. We conveniently designated near-complete and incomplete TME as “inappropriate” mesorectal excision in contrast with complete mesorectal excision, as previously described [11]. Additional excision was considered for tumors with high-risk mesorectal fascia, consisting primarily of threatened mesorectal fascia (c/pT4) and suspicious invasion of the adjacent viscera. Concurrent diversional ileostomy was installed depending on the surgeon’s discretion, particularly in patients who have undergone preoperative chemoradiotherapy and a complicated procedure. All operations were performed by a senior colorectal surgeon sufficiently experienced with robot approaches. The entire procedure, with particular attention to TME extent and quality, was reexamined through video records and gross photographs.
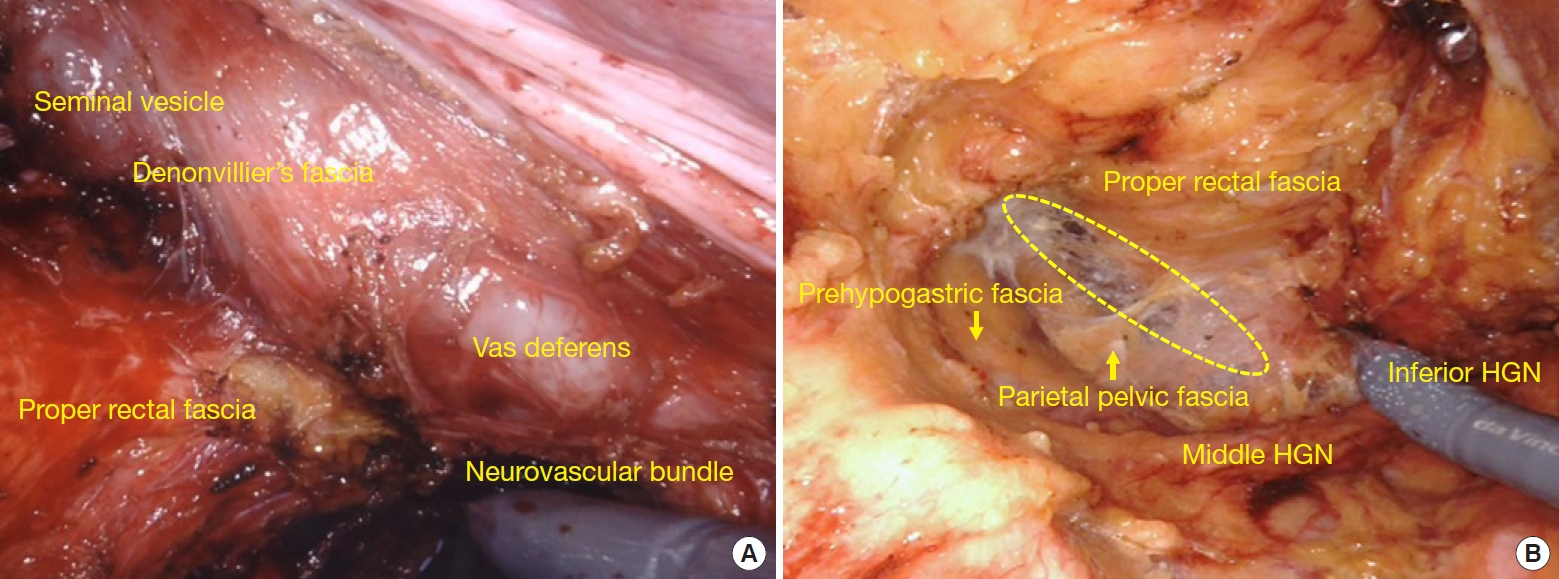
Total mesorectal excision (TME) and related pelvic fascial structure. (A) Anterior and anterolateral pelvic cavity. (B) Posterior pelvic cavity. The area inside an ellipse indicates the TME dissection plane between the proper rectal and prehypogastric fascia, shown as a pasteboard-like fusion. HGN, hypogastric nerve.
Postoperative evaluations
All patients were followed up periodically every 3 to 6 months for the first 5 postoperative years, and every 2 years thereafter. The severity of surgical complications was evaluated using the Clavien-Dindo classification (https://drcesarramirez.com/). Anorectal function was analyzed in patients aged ≤ 65 years by measuring fecal incontinence score according to Wexner’s method(https://www.mdcalc.com/) and by high-resolution anorectal manometry (complete performance rate, 93.7%). Incontinence and manometric parameters, including mean resting pressure (MRP), maximal squeezing pressure (MSP), urge to defecate volume (UDV), and maximal tolerance volume (MTV), were examined preoperatively, and 6–12 and 12–24 months after primary and restorative surgery, respectively. Recurrence/survival > 5 years after surgery and 2-year functional outcomes were assessed in propensity-score matched groups (Table 2). Median follow-up period was 57 months (interquartile range [IQR], 27–82 months).
Statistical analyses
To reduce potential bias caused by confounding, the 2 groups were subjected to 1:1 propensity-score matching using the nearest-neighbor method (Table 2). Clinicopathological and operative features were compared using 2-tailed Fisher exact tests, and continuous variables were compared using paired Student t-tests. Potential associations between operation-associated and prognostic outcomes were reanalyzed by binomial logistic regression. OS and DFS were analyzed using the Kaplan-Meier method and compared by the log-rank test, and significant variables were validated using a Cox proportional-hazards regression model. All statistical analyses were performed using IBM SPSS Statistics ver. 21 (IBM Corp., Armonk, NY, USA), with P< 0.05 defined as statistically significant. All results were calculated for the propensity-score matched groups, unless otherwise specified.
RESULTS
Overall clinicopathological features in all patients undergoing PME or TME
Clinicopathological and operative findings were compared in the PME and TME groups (Table 1), and several differences of all patients were detailed. Complete mesorectal excision was achieved in 617 of the 671 patients (92.0%), with no difference between the PME and TME groups. Comparisons of pinned and formalin-fixed samples from the PME and TME groups showed no significant differences in DRM length (36 mm vs. 34 mm, P= 0.189), proximal resection margin (83 mm vs. 88 mm, P= 0.070), and total lymph node harvest (21 vs. 20, P= 0.377). Low ligation was performed mostly to excise the IMA (94.9%) in both groups. Additional excision of adjacent viscera was required to achieve R0 resection in 41 patients, with no difference between the groups. Partial excision of Denonvilliers and parietal pelvic fascia, en bloc with the rectum and mesorectum, was most frequent (65.9%). Postoperative complication rate was somewhat higher in the TME than in the PME group (21.4% vs. 14.5%, P= 0.027), but did not differ by severity grade. Anastomotic leakage did not differ in the PME and TME groups, whereas stricture rate tended to be higher in the TME group (0.5% vs. 2.1%, P= 0.054). No patient in either group died ≤ 1 month after surgery. Several parameters that differed in the PME and TME groups were no longer different after propensity-score matching, except for DRM length (P= 0.001), the transient diversion rate (P= 0.001), and hospitalization (P=0.007) remained greater in the TME group. Fewer than 1/3 of propensity-score matched patients received either neoadjuvant treatment or postoperative chemoradiotherapy, with no difference between the 2 groups. Of the 316 patients with middle rectal cancer, 137 (43.4%) and 179 (56.6%) underwent PME and TME, respectively. The mean length of the anorectal canal in 200 consecutive patients was found to be 3.2 cm (range, 2–5 cm), with no difference between male and female patients.
Cumulative recurrences in the propensity-score matched partial and total mesorectal excision groups
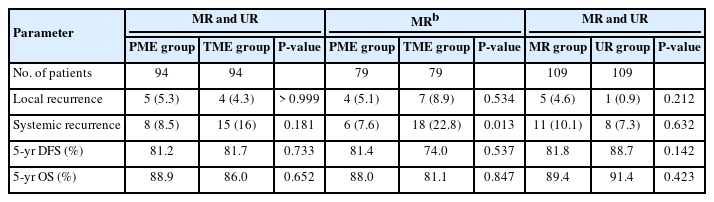
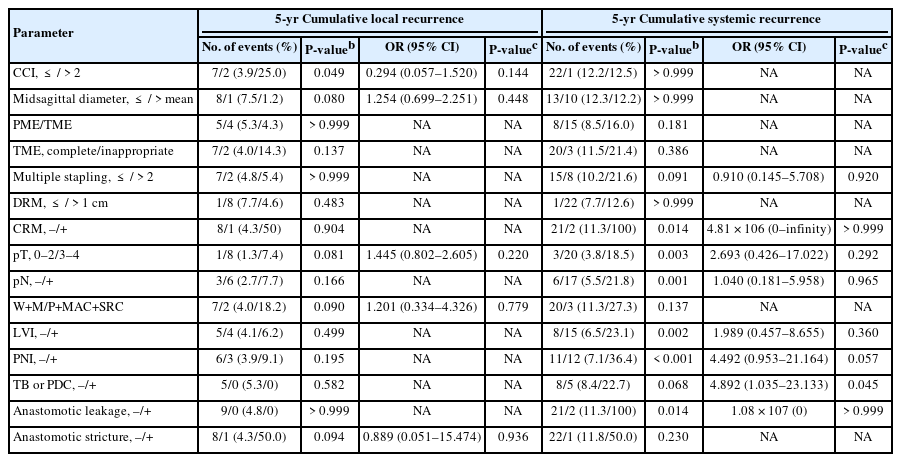
In the total propensity-score matched cohort, the 5-year cumulative local and systemic recurrence rates were 4.8% and 12.2%, respectively. Comparative recurrences in propensity-score matched patients are shown in Table 3. Local (P> 0.999) and systemic (P= 0.181) recurrence rates in patients with middle/upper rectal cancer did not differ between the PME and TME groups. Although local (5.1% vs. 8.9%, P= 0.534) recurrence rates in patients with middle rectal cancer did not differ in the PME and TME groups, systemic recurrence rates differed significantly in these groups (7.6% vs. 22.8%, P= 0.013). The rate of systemic recurrence was about 5-fold higher in patients with local recurrence than those without it (55.6% vs. 10.1%, P= 0.002). Increased Charlson comorbidity index (score, > 2) was associated with local recurrence (P= 0.049) (Table 4). Systemic recurrence was associated with perineural invasion (PNI; P< 0.001), lymphovascular invasion (LVI; P= 0.002), pN+ (P= 0.001), advanced pT (≥ 3; P= 0.003), CRM+ (P= 0.014), and anastomotic leakage (P= 0.014). Notably, tumor budding (TB) or poorly-differentiated cluster (PDC) independently increased the rate of systemic recurrence (odds ratio [OR], 4.892; P= 0.045) (Table 4). Additional excision of PME and TME did not affect either the local or systemic recurrence rate (P> 0.999). The 5-year cumulative local (3.0% [P= 0.445] to 5.1% [P> 0.999]) and systemic (8.5% [P= 0.112] to 17.2% [P= 0.865]) recurrence rates did not differ by the DRMs, classified by cutoffs of 2, 3, and 4 cm, in all patients with middle/upper rectal cancer, regardless of pStages (0–II [P= 0.397] vs. III–IV [P= 0.899]) (Table 5).
Cumulative survival outcomes in the propensity-score matched partial and total mesorectal excision groups
Survival outcomes of propensity-score matched patients are shown in Table 3 and Fig. 3. The 5-year cumulative DFS (P = 0.733) and OS (P= 0.652) rates were similar in patients with middle/upper rectal cancer who underwent PME and TME, as were the 5-year DFS (81.4% vs. 74.0%, P= 0.537) and OS (88.0% vs. 81.1%, P= 0.847) rates in patients with middle rectal cancer. PNI independently reduced both DFS (hazard ratio [HR], 2.976; P= 0.008) and OS (HR, 3.25; P= 0.002) (Table 6). Anastomotic leakage (HR, 16.483; P= 0.001) and unfavorable differentiation (poorly differentiated, mucinous, or signet-ring cell; HR, 2.936; P < 0.050) were independently associated with reduced DFS, whereas inappropriate TME (HR, 2.839; P< 0.050) and older age ( > mean; HR, 2.144; P < 0.050) were independently associated with reduced OS. Increased Charlson comorbidity index, CRM+, and pN+ were correlated with reduced DFS (P < 0.050). The 5-year cumulative DFS and OS rates were not affected by DRM cutoffs, as were found in recurrence outcomes (Table 5).

Cumulative 5-year DFS (A–C) and OS (D–F) rates in propensity-score matching patients with (A, D) middle rectal cancer undergoing PME vs. TME, (B, E) patients with middle and upper rectal cancer undergoing PME vs. TME, and (C, F) patients with middle vs. upper rectal cancer. DFS, disease-free survival; MR, middle rectum indicating >5 to 10 cm from the anal verge; PME, partial mesorectal excision; TME, total mesorectal excision; UR, upper rectum indicating >10 to 15 cm from the anal verge; OS, overall survival.
Anorectal functional changes in the propensity-score matched partial and total mesorectal excision groups
Fecal incontinence score and principal manometric measurements did not differ between the PME and TME groups, except that MTV was lower in the TME than in the PME group until 6 to 12 months (P= 0.004), and UDV was lower in the TME group until 12 to 24 months (P= 0.045) (Fig. 4). In both groups, incontinence scores were higher at 12 to 24 than at 6 to 12 months (6.6 vs. 3.7, P< 0.001). Principal manometry values were also lower postoperatively than preoperatively (P< 0.001), except for MSP (P= 0.086), but all postoperative values were within the IQR of the healthy population [13]. Possible parameters associated with anorectal dysfunction were examined 12 to 24 months after surgery in middle/upper rectal cancer (Table 7). Incontinence was independently associated with TME (OR, 2.009; P< 0.050), older age (OR, 4.366; P< 0.001), and prolonged operation time (OR, 2.196; P< 0.050). Reduced MRP and MSP were associated with older age and female sex, respectively (P< 0.001 each), while UDV and MTV were associated with prolonged operation time and tumor location in the middle rectum, respectively (P< 0.040 each).
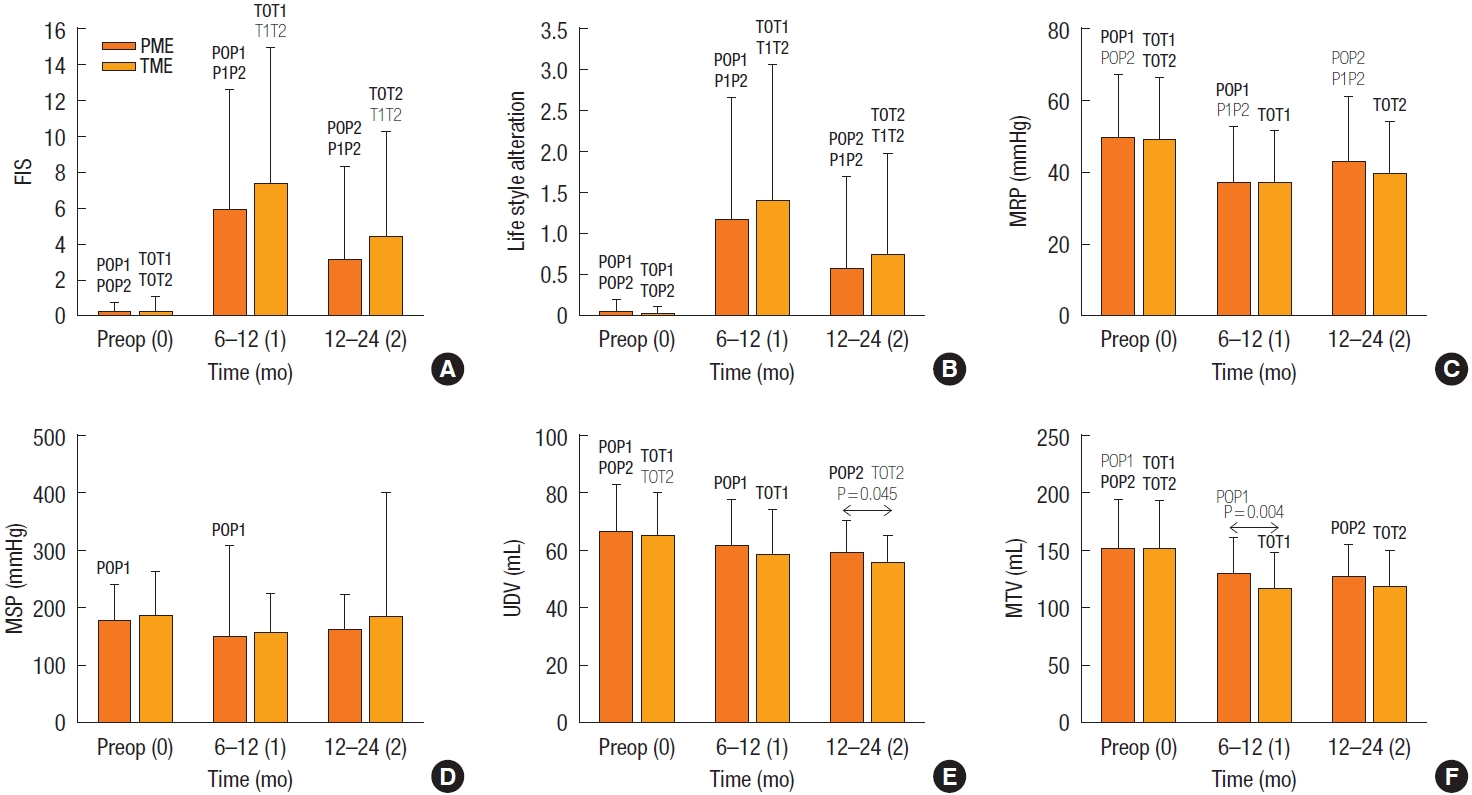
Anorectal function measurement. FIS (the sum of solid, liquid, gas, pad use, and lifestyle alteration) (A), lifestyle alteration (B), and 4 principal manometric parameters (C–F), at baseline 6 to 12 and >12 to 24 months after surgery in the PME and TME groups. Periods “0–2” mean values (mean±standard error of mean) at baseline, 6 to 12 months, and >12 to 24 months, respectively, concurrent with P0P1, showing P-values for comparisons between PME period 0 vs. 1, equally applied for TME. FIS, fecal incontinence score; Preop, preoperative; PME, partial mesorectal excision; TME, total mesorectal excision; MRP, mean resting pressure; MSP, maximal squeezing pressure; UDV, urge to defecate volume; MTV, maximal tolerance pressure. Each bar shows the mean±standard deviation. Bold font, P>0.001; plain font, P=0.001 to P=0.05.
DISCUSSION
Recurrence and survival rates in patients with middle/upper rectal cancer did not differ between those who underwent PME and TME in this study, confirming equivalent prognostic outcomes of the 2 procedures. Prognostic outcomes were consistent with a previous study involving 147 patients who underwent PME alone and the other review [4, 5]. Although the present study found that local recurrence rates in patients with middle rectal cancer were somewhat higher in the TME than in the PME group, the differences were not statistically significant. However, the systemic recurrence rate was significantly higher in the TME group. Few oncologically unfavorable parameters were observed in the TME group, except for greater tendency of anastomotic stricture and high rate of general morbidity [14]. Because the present study found that local recurrence was a potent predictor (5-folds) of systemic recurrence, a slightly higher local recurrence rate may be arithmetically amplified to yield a higher systemic recurrence rate in the TME group. On the other hand, type I error may somewhat affect an exaggerated difference in systemic recurrence rates between the 2 groups. These recurrence outcomes were not translated into any survival differences between the 2 procedures.
Biological traits of tumor deposits have been variably designated, for example, either tumor deposits (N1c) or mesorectal tumor microfoci [15]. These deposits are indicative of oncological aggressiveness, causing local recurrence and poorer prognosis, suggesting that these components can be combined into a unified category as ATDs. Thus, LVI/PNI, TB/PDC, extramural vascular invasion, and extranodal extension can be regarded as ATDs, based on their histological characteristics and biological behavior [16-21]. Systemic recurrence has been associated with PNI, LVI, and especially with TB/PDC in the present study. TB and PDC are regarded as powerful predictors of survival outcomes, outperforming the AJCC stage and other adverse features [18]. LVI and PNI are predictors of metastatic disease and recurrence with reduced survival, and have therefore been designated as category I prognostic indicators in the AJCC cancer staging. Inappropriate TME resulted in an HR of 2.8 for OS, in agreement with a previous Dutch TME trial and a Norwegian audit [22, 23]. All these findings emphasize the importance of en bloc excision with mesorectal fascia to achieve complete mesorectal excision, eliminating a substantial part of ATDs.
In this study, systemic recurrence was associated with CRM+ and advanced pT or pN+, whereas CRM+ and pN+ were correlated with reduced DFS. In line with these findings, showing that CRM+ correlated with systemic recurrence or DFS, 1 study suggested that CRM involvement is an indicator of advanced disease rather than inadequate local surgery because CRM+ patients die of distant disease before local recurrence [24]. Practically, the risk of CRM+ increases with more advanced T and N categories, making CRM+ a powerful predictor of distant metastases and reduced survival [3, 25]. The present study also found that unfavorable histology was an independent factor reducing DFS, in line with previous studies [26, 27]. Older age (> mean) independently correlated with reduced OS (HR, 2.144), whereas increased Charlson comorbidity index (> 2) was associated with increased local recurrence and reduced DFS. These 2 parameters seem closely correlated with reduced survival, such that elderly patients with rectal cancer are at higher risk of treatment-related complications and comorbidities [28, 29].
Although this study did not evaluate measurable advantages of robot procedure, mechanistic advantages facilitate complete mesorectal excision, particularly for accurate mesofascial dissection preserving rectal and pelvic neurovascular bundles. Additionally, the robotic vessel sealer efficiently provides a circular and smooth cut surface of the distal mesorectum through effective hemostasis and clear amputation. Distal mesorectal excision must be accurately performed to secure a smooth mesorectal cut surface perpendicular to the rectal wall because an irregular mesorectal end can result in a severe mesorectal defect or insufficient bulk. The lack of correlation between additional excision and recurrence rate in the present study needs to be interpreted with caution, as only patients with advanced pT4 tumors underwent additional excision. Compared with a less extensive operation, more extensive surgery has been found to reduce the risk of recurrence in patients with advanced tumors [30].
Because TME has been associated with increased rates of anastomotic complications and anorectal dysfunction, PME according to tumor level is regarded as a reasonable surgical approach [3, 5, 6, 8]. Similarly, the present study found that incontinence was independently associated with TME (OR, 2.009) compared with PME, along with older age and prolonged operation time. Notably, the total length of the resected specimen and lymph node harvest did not differ significantly between patients who underwent PME and TME, suggesting equivalent oncological resection. Prognostic outcomes did not differ among the 3 levels of DRM (2, 3, and 4 cm) regardless of pStages, in accordance with previous findings, and recently the American Society of Colon and Rectal Surgeons recommended a distal mural resection margin of 2 cm in their current guideline [3, 30, 31]. Although initial studies exhibited 4 to 5 cm distal mesorectal spread [8] which had been shown as intramural spread deposits from approximately 3 to 4 cm of the primary tumor. Taken together, the present results indicate that PME may be an optimal choice for patients with middle rectal cancer with a lower margin of > 5 cm from anal verge, maintaining a DRM of 2 to 4 cm, except for tumors with unfavorable histology, high-risk mesorectal fascia, and pT4b or pN2 [12, 30, 32].
This study had several limitations. First, it was a retrospective, single-center, case-series analysis, suggesting a possible selection bias caused by customary implementation of PME or TME, with surgical procedure not chosen by absolute indication for middle rectal cancer. We attempted to minimize bias by reexamination of visual records and by propensity-score matching using multivariate analyses. Second, comparative groups could not be perfectly propensity-score matched for recurrence and survival analyses, due to limited subjects of single institutional study. However, parameters potently associated with prognostic outcomes, namely, clinical stage and neoadjuvant treatment, were primarily matched to compare the PME and TME groups.
In conclusion, the findings of this study suggest that complete PME may be objectively valid in the treatment of middle rectal cancer based on oncological and functional outcomes. PME for middle rectal cancer can result in competent prognostic outcomes and lower morbidity rates, including lower rates of anastomotic complications and anorectal dysfunction. Although TME may sometimes induce intractable complications or anorectal dysfunction, It can alternatively be chosen for tumors with a lower margin of ≤ 5 cm from the anal verge, particularly in advanced T4 or N2 category and extensive ATDs. Randomized control trials comparing PME and TME in a sufficient number may confirm findings optimizing the use of PME in treating middle rectal cancer.
Notes
CONFLICT OF INTEREST
Park In Ja is the current editor-in-chief of Annals of Coloproctology; however, she did not interfere with the reviewing or decision process of this manuscript. No other potential conflict of interest relevant to this article was reported.
FUNDING
None.
AUTHOR CONTRIBUTIONS
Conceptualization: EJK, CWK, JCK. Data curation: all authors. Formal analysis: EJK, CWK, JLL, YSY. Investigation: EJK, CWK, JCK. Methodology: EJK, CWK, YSY, IJP, JCK. Project administration: EJK, CWK, JCK. Resources: JCK, JLL. Validation: EJK, CWK, JLL, YSY, IJP, SBL, CSY. Writing–Original Draft: EJK, CWK, JCK. Writing–Review & Editing: all authors.
All authors have read and approved the final manuscript.