Long-term clinical outcomes after high and low ligations with lymph node dissection around the root of the inferior mesenteric artery in patients with rectal cancer
Article information
Abstract
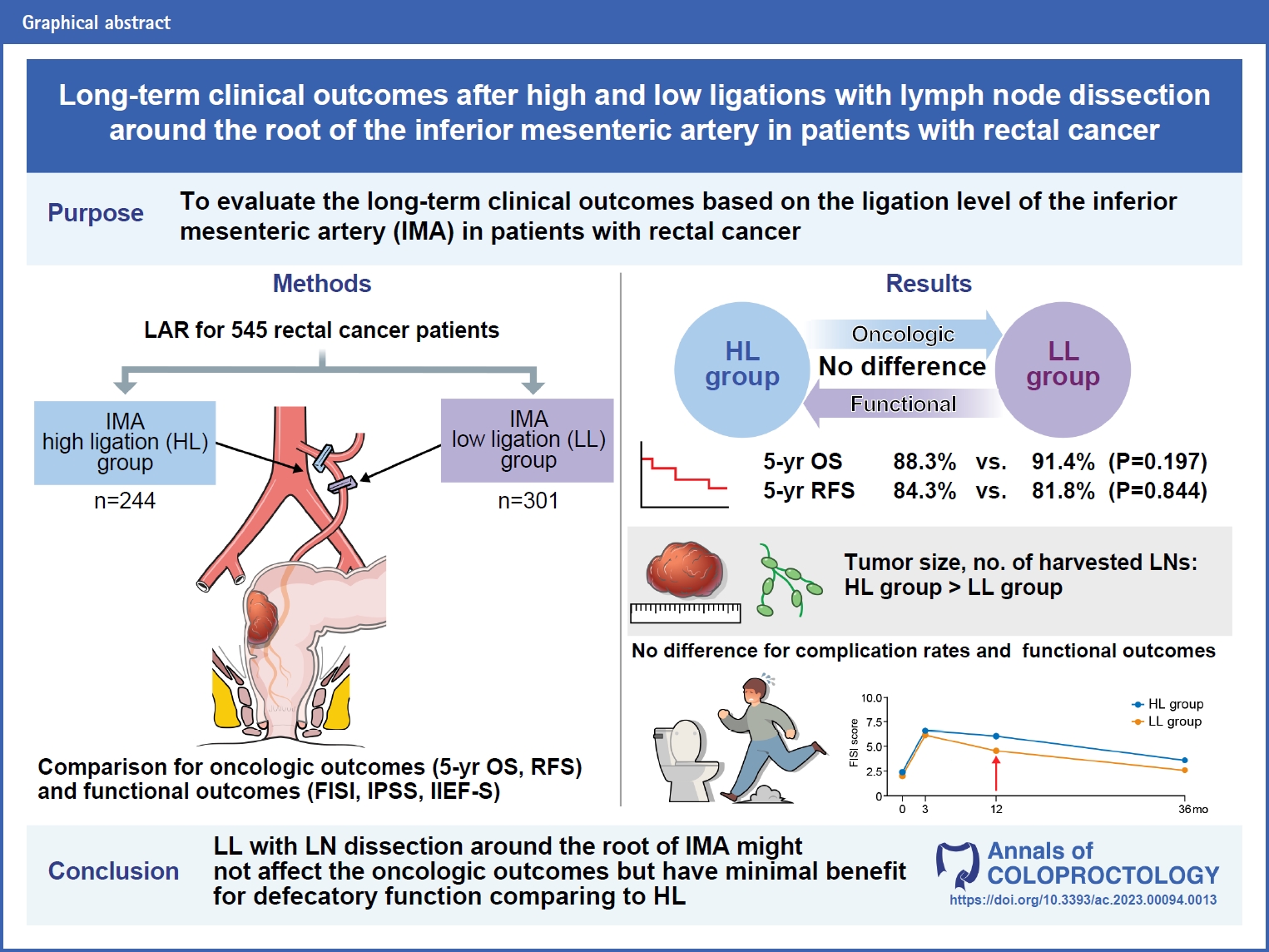
Purpose
This study aimed to evaluate the long-term clinical outcomes based on the ligation level of the inferior mesenteric artery (IMA) in patients with rectal cancer.
Methods
This was a retrospective analysis of a prospectively collected database that included all patients who underwent elective low anterior resection for rectal cancer between January 2013 and December 2019. The clinical outcomes included oncological outcomes, postoperative complications, and functional outcomes. The oncological outcomes included overall survival (OS) and relapse-free survival (RFS). The functional outcomes, including defecatory and urogenital functions, were analyzed using the Fecal Incontinence Severity Index, International Prostate Symptom Score, and International Index of Erectile Function questionnaires.
Results
In total, 545 patients were included in the analysis. Of these, 244 patients underwent high ligation (HL), whereas 301 underwent low ligation (LL). The tumor size was larger in the HL group than in the LL group. The number of harvested lymph nodes (LNs) was higher in the HL group than in the LL group. There were no significant differences in complication rates and recurrence patterns between the groups. There were no significant differences in 5-year RFS and OS between the groups. Cox regression analysis revealed that the ligation level (HL vs. LL) was not a significant risk factor for oncological outcomes. Regarding functional outcomes, the LL group showed a significant recovery in defecatory function 1 year postoperatively compared with the HL group.
Conclusion
LL with LNs dissection around the root of the IMA might not affect the oncologic outcomes comparing to HL; however, it has minimal benefit for defecatory function.
INTRODUCTION
In 2020, the World Health Organization (WHO) announced the incidence of colorectal cancer as the 3rd highest after breast and lung cancers, and the mortality rate of colorectal cancer was second after that of lung cancer [1].
According to the guidelines published by the National Comprehensive Cancer Network (NCCN) in 2022, the standard treatment for rectal cancer is to remove the primary tumor by securing an appropriate circumferential resection margin and distal resection margin (DRM), followed by total mesorectal excision (TME) [2]. In addition, inferior mesenteric artery (IMA) ligation is a necessary process during TME, and it can be divided into high ligation (HL) and low ligation (LL) depending on the location of the ligated artery. However, the NCCN guidelines do not cover the location of IMA ligation, and the debate on the location of IMA ligation has been ongoing since Moynihan [3] and Miles [4] introduced HL and LL in 1908, respectively.
HL is an IMA ligation method. Lymph node (LN) dissection from the periphery is performed by ligating the root of the IMA branching from the aorta. Laparoscopic surgery is the most widely used surgical method for treating rectal cancer. In this laparoscopic view, many surgeons prefer HL because the root of the IMA is easily accessible, and it is relatively easier than LL because only the root of the IMA branching from the aorta must be identified and ligated. Furthermore, by performing lymphadenectomy around the origin of the IMA during the ligation process, D3 lymphadenectomy, defined by the Japanese Society for Cancer of Colon and Rectum (JSCCR), is possible in HL [5]. In addition, it is possible to secure a longer mesenteric length in HL than in LL when performing anastomosis in the pelvic cavity [6, 7]. However, a significant reduction in blood flow to the anastomosis site is observed in HL compared with LL, and the length of the proximal colonic denervation is longer in HL [8, 9]. In addition, there is a high possibility of damage to the superior hypogastric plexus passing through the left and right sides of the origin of the IMA when performing HL [10]. LL can be more complicated than HL because the IMA, left colonic artery, and superior rectal artery must be identified before ligation. In addition, LN dissection is usually performed around the superior rectal artery in LL, unlike HL; however, it has recently been possible to dissect the LN from the origin of the IMA [11].
Furthermore, several debates have arisen owing to the anatomical differences between the 2 methods, which are still ongoing. First, it is thought that HL can improve oncological outcomes compared with LL because LN dissection is performed at the IMA origin, and more LNs can be harvested. Slanetz and Grimson [12] and Singh et al. [13] reported that HL was more oncologically beneficial in patients with advanced colorectal cancer. However, Yasuda et al. [14], AlSuhaimi et al. [15], and Park et al. [16] reported no significant difference in oncological outcomes between the 2 methods. Regarding the occurrence of postoperative complications, several studies have reported that ischemic colitis, necrosis, and anastomotic leak frequently occur in HL due to reduced blood flow to the anastomosis [8, 17–19]; however, other studies have reported that no difference exists in the occurrence of complications between the 2 methods, while others have indicated that HL could be a safer and more appropriate option [16, 20–22]. Regarding postoperative functional outcomes, the possibility of damage to defecatory and urogenital functions may be higher in HL than in LL because of the high possibility of proximal colonic denervation and superior hypogastric plexus damage in HL. However, some studies have reported no differences in functional outcomes between the 2 methods [23, 24]. Therefore, this study aimed to evaluate the long-term clinical outcomes based on the ligation level of IMA.
METHODS
Ethics statement
This study was conducted in compliance with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of the National Cancer Center of Korea (No. NCC2022-0367). The requirement for informed consent was waived due to the retrospective nature of the study.
Study design and patients
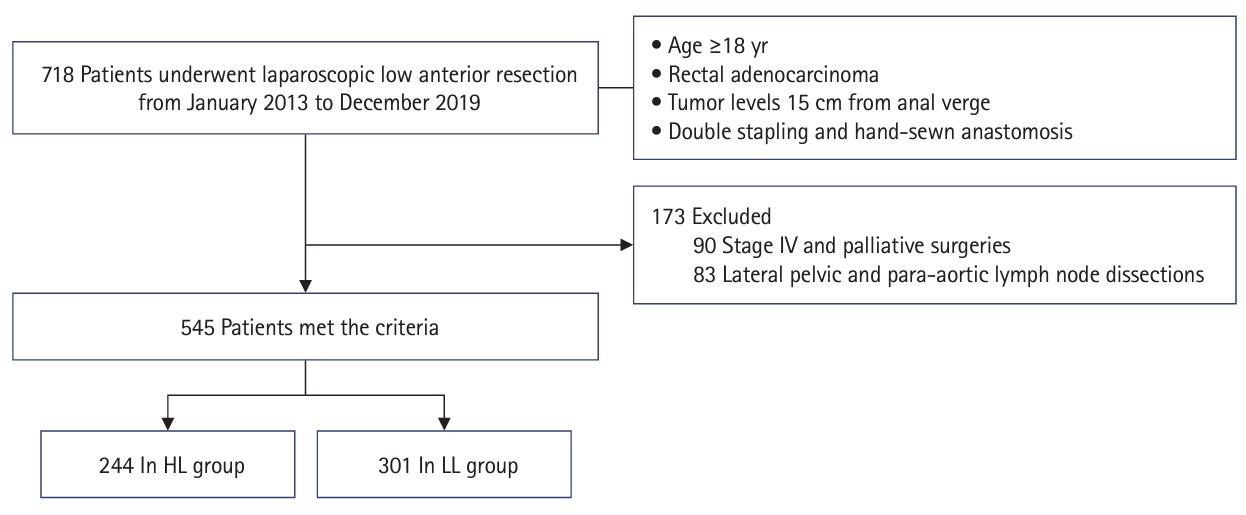
This retrospective study was conducted on patients aged ≥18 years who underwent laparoscopic low anterior resection for rectal adenocarcinoma between January 2013 and December 2019. Patients who underwent resection of other major organs, such as hepatectomy or palliative surgery at stage IV, and those who underwent lateral pelvic and para-aortic LN dissections were excluded from this study. A total of 545 patients were included: 244 patients underwent HL, and 301 patients underwent LL (Fig. 1). Their medical records were retrospectively reviewed for clinicopathological data, survival rates, complications, and functional outcomes, according to the ligation method.
Perioperative management
For preoperative clinical staging, colonoscopy, abdominal and pelvic computed tomography (CT), chest CT, and rectal magnetic resonance imaging (MRI) were performed for all patients. If the tumor was located within 10 cm from the anal verge and the clinical category was T3/T4 or node-positive, neoadjuvant chemoradiotherapy (50.4 Gy radiotherapy and capecitabine bid 825 mg/m2 on radiotherapy day) was performed before radical surgery. According to the colorectal cancer follow-up protocol of the National Cancer Center of Korea, chest and abdominal and pelvic CTs were performed every 6 months for 5 years, and colonoscopy was performed at 1, 3, and 5 years postoperatively. Additional radiologic examinations such as positron emission tomography-CT or MRI were performed as needed during follow-up.
Surgical techniques
All patients underwent laparoscopic low anterior resection and radical lymphadenectomy around the origin of the IMA performed by experienced surgeons at the Colorectal Cancer Center of the National Cancer Center of Korea. The operation was mainly performed by 2 surgeons. One surgeon mainly performed LL but selectively performed HL only in cases where LNs were conglomerated at the origin of the IMA and the left colonic artery could not be preserved due to anatomical variation or technical failures such as hemorrhage (HL, 20 patients; LL, 224 patients). The other surgeon selectively performed LL for early cancer but mainly performed HL for advanced rectal cancer (HL, 156 patients; LL, 27 patients). HL was performed using a metal clip or hemo-lock clip 1 to 2 cm distal from the aorta at the origin of the IMA. The inferior mesenteric vein and left colic artery were ligated using metal clips at the same level as the HL or at the inferior border of the pancreas. In LL, the superior rectal artery was ligated using a metal clip or hemo-lock clip. However, the left colic artery was preserved, and the inferior mesenteric vein was ligated using a metal clip at the LL level. TME was performed in both groups, and the sacral splanchnic nerve, hypogastric nerve, and pelvic plexus were well preserved. The adequacy of blood flow in the marginal artery or vasa recta of the proximal colon was confirmed visually or by palpation. Anastomosis between the proximal colon and distal rectum or anus was performed using instrumental double-stapling or coloanal hand-sewn anastomosis. An air-leak test was performed after anastomosis, and additional sutures were placed when an air bubble was observed. Splenic flexure mobilization was selectively performed when the anastomosis was under tension. Diverting loop ileostomy was selectively performed when chemoradiotherapy was administered preoperatively or when anastomosis was close to the anus.
Oncological outcomes measurement
The 5-year overall survival (OS) and relapse-free survival (RFS) rates were evaluated to analyze oncological outcomes. OS was defined as the period from surgery to death, whereas RFS was defined as the period from surgery to recurrence. Recurrence was defined as a case in which a lesion that gradually increased in size was discovered after the primary tumor was removed and diagnosed by a radiologist or pathologically through a biopsy. Local recurrence is defined as any recurrence occurring within the true pelvis. It is classified into 4 types according to the location of recurrence in the true pelvis: anterior, posterior, lateral, and anastomosis site. In addition, systemic recurrence was defined as recurrence occurring beyond the true pelvis.
Postoperative complication measurement
Postoperative complications were only included if they occurred within 30 days postoperatively; however, ischemic colitis was included if it was detected using colonoscopy, which was performed as a follow-up protocol. Surgical site infection (SSI) was classified into superficial and deep SSI. Superficial SSI was defined as a case in which erythema, tenderness, seroma, and pus discharge were observed at the skin and subcutaneous levels. Deep SSI was defined as a case in which wound dehiscence occurred as the infection invaded the fascia and muscle layers. Postoperative bleeding was classified as anastomosis site or intra-abdominal bleeding. Anastomosis site bleeding was defined as active bleeding observed on sigmoidoscopy, and hemostasis was performed using a metal clip. Intra-abdominal bleeding was defined as the presence of prominent bleeding or hematoma on a CT scan. Ileus was defined as a case in which insertion of an nasogastric tube or surgical intervention was performed for symptoms such as nausea and vomiting, and obstruction was observed on imaging. Anastomotic leakage was defined as grade B or C, as defined by Rahbari et al. [25], when communication between the intraluminal and extraluminal compartments of the anastomosis site was observed on CT imaging, along with clinical symptoms such as fever, leukocytosis, and turbid color of drainage. Urinary retention was defined as a case in which the Foley catheter was removed and reinserted because of urinary difficulty during hospitalization. Ischemic colitis was defined as a case with accompanying symptoms confirmed using a colonoscopy.
Functional outcome measurement
Functional outcomes were evaluated using questionnaires administered preoperatively and 3, 12, and 36 months postoperatively. Defecatory, urinary, and male sexual functions were evaluated. Bowel function was evaluated using the Fecal Incontinence Severity Index (FISI), urinary function using the International Prostate Symptom Score (IPSS) in both male and female patients, and male sexual function using the International Index of Erectile Function (IIEF-5). The change in questionnaire scores over time postoperatively was assessed to determine whether there was a difference in functional outcomes between the 2 groups.
Statistical analysis
Categorical variables are expressed as frequencies with percentages and the chi-square or Fisher exact tests were used to compare 2 groups. For continuous variables, the 2 groups were compared using 2 sample t-test or Wilcoxon rank sum test and expressed as medians and ranges. The 5-year OS and RFS and survival curves were estimated using the Kaplan-Meier method. Comparison of the 2 survival curves was tested using the log-rank test. The risk factors for the OS and RFS were analyzed using the Cox proportional hazard model. The difference between the 2 groups according to the difference in time was analyzed using the Wilcoxon rank sum test and mixed-effect model to analyze the functional outcome. Statistical significance was set at P<0.05. All statistical analyses were performed using R ver. 4.2.0 (R Foundation for Statistical Computing).
RESULTS
Clinicopathologic characteristics
There were 244 and 301 patients in the HL and LL groups, respectively. There were no significant differences in sex, age, body mass index, comorbidities, preoperative chemoradiotherapy, tumor level, pathologic stage, intraoperative bleeding, operation time, and diverting ileostomy between the 2 groups. However, the tumor size was larger in the HL group than in the LL group (P=0.006). The number of harvested LNs was higher in the HL group than in the LL group (P=0.002). However, when LNs harvest adequacy was compared based on the evaluation criteria of 12, there was no difference between the 2 groups (P=0.260). The length of the proximal resection margin (PRM) was longer in the HL group (P<0.001), whereas the length of DRM was longer in the LL group (P<0.001). Splenic flexure mobilization was performed more frequently in the HL group (P=0.004) (Table 1).
OS and RFS
The average follow-up period for survival was 56 months. There was no significant difference in the 5-year OS rate (88.3% vs. 91.4%, P=0.197) and RFS rate (84.3% vs. 81.8, P=0.844) between the 2 groups (Fig. 2). Multivariate analysis of factors affecting OS and RFS revealed that male sex (hazard ratio [HR], 2.154; 95% confidence interval [CI], 1.085–4.275; P=0.028), T3–4 stage (HR, 2.326; 95% CI, 1.217–4.446; P=0.011), nodal metastasis (HR, 1.945; 95% CI, 1.128–3.353; P=0.017), and comorbidity (HR, 1.948; 95% CI, 1.118–3.395; P=0.019) were associated with OS, whereas T3–4 stage (HR, 2.323; 95% CI, 1.322–4.083; P=0.003), nodal metastasis (HR, 4.891; 95% CI, 2.825–8.469; P<0.001), operation time (HR, 1.313; 95% CI, 1.063–1.621; P=0.011), and adjuvant treatment (HR, 0.396; 95% CI, 0.228–0.686; P=0.001) were associated with RFS. However, Cox regression analysis revealed that the ligation level (HL vs. LL) was not a significant risk factor for OS or RFS (Table 2).

Overall and relapse-free survivals of high ligation (HL) and low ligation (LL) groups. (A) Five-year overall survival (HL group [88.3%] vs. LL group [91.4%], P=0.197). (B) Relapse-free survival (HL group [84.3%] vs. LL group [81.8%], P=0.844). IMA, inferior mesenteric artery.
Postoperative complication
Postoperative complication occurred in 104 (19.1%) of the 545 patients, of which 47 (45.2%) were in the HL group and 57 (54.8%) were in the LL group; however, there was no significant difference between the 2 groups. The most common complication was ileus (HL group [4.9%] vs. LL group [6.6%]), followed by anastomotic leakage (HL group [4.5%] vs. LL group [4.3%]) and urinary retention (HL group [3.7%] vs. LL group [3.0%]). There were no significant differences in ileus, anastomotic leakage, or urinary retention between the 2 groups. Ischemic colitis occurred in 3 patients in the HL group and 1 in the LL group; however, there was no significant difference between the 2 groups. Postoperative bleeding occurred in 1 patient (intra-abdominal bleeding) in the HL group and 5 (anastomotic site bleeding in 3 and intraabdominal bleeding in 2) in the LL group; however, there was no significant difference between the 2 groups (Table 3).
Recurrence pattern of patients
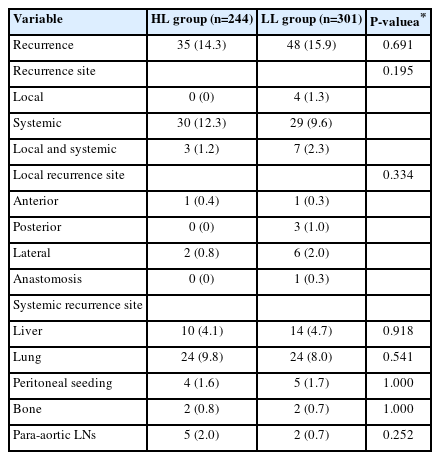
Recurrence occurred in 83 of the 545 patients (15.2%), of which 35 (14.3%) were in the HL group, and 48 (15.9%) were in the LL group. Local recurrence occurred in 11 of the 545 patients (2.6%; HL group, 3 patients [1.2%]; LL group, 11 patients [3.6%]), and systemic recurrence occurred in 69 patients (12.7%; HL group, 33 patients [12.5%]; LL group, 36 patients [11.9%]). The most frequent site of local recurrence was the lateral side (HL group, 2 patients [0.8%]; LL group, 6 patients [2.0%]), and the most frequent site of systemic recurrence was the lungs (HL group, 24 patients [9.8%]; LL group, 24 patients [8.0%]). There were no significant differences in the recurrence patterns between the 2 groups (Table 4).
Functional outcomes
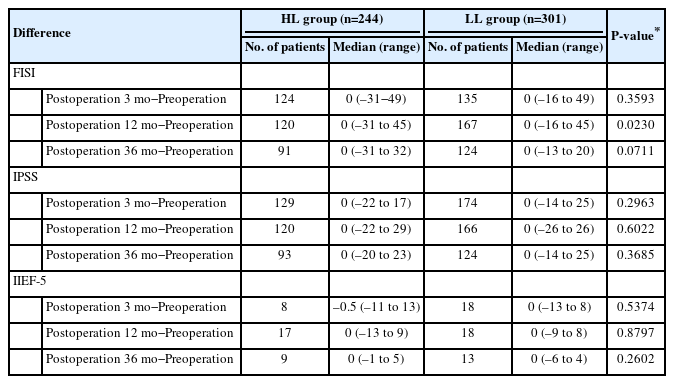
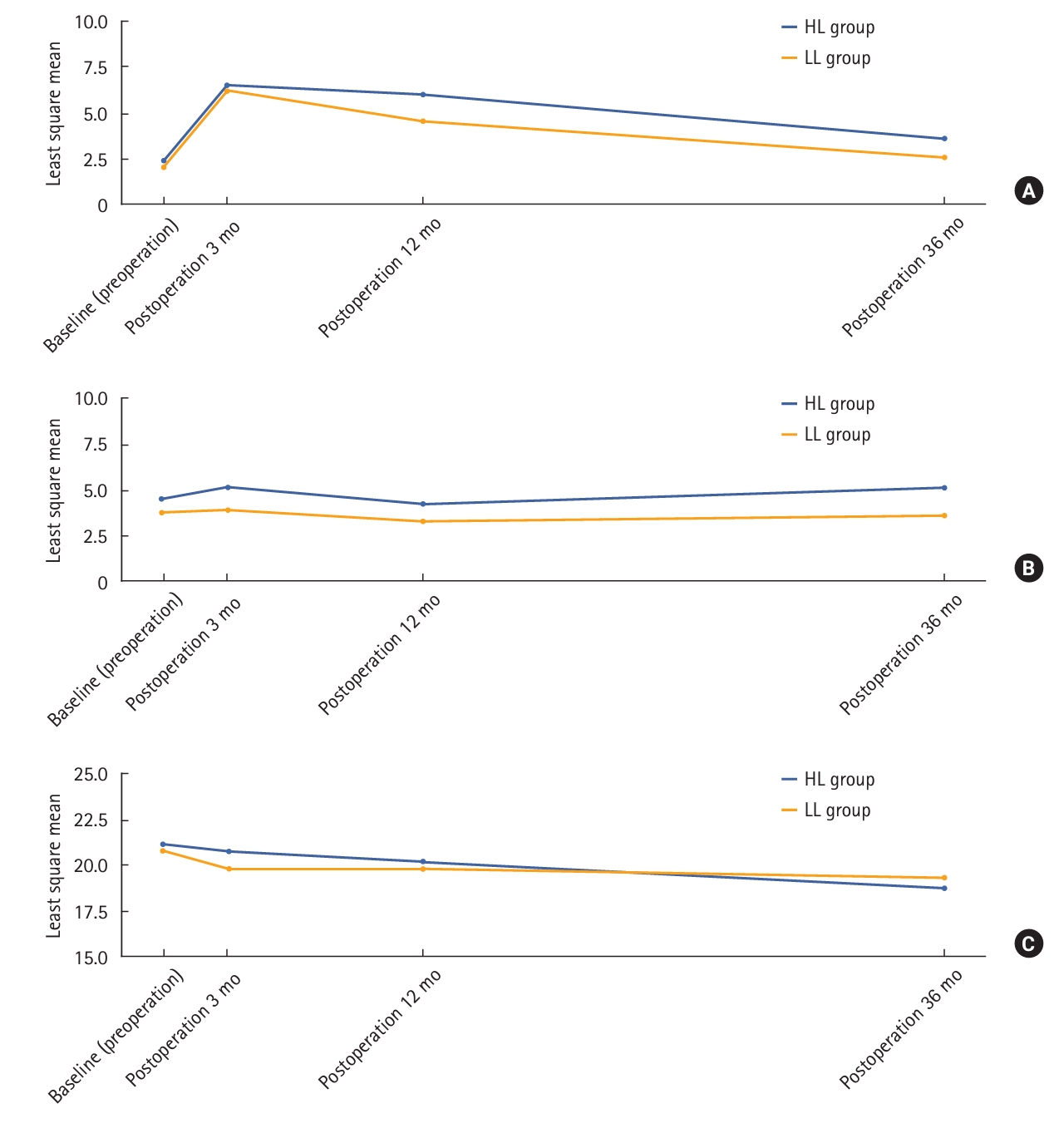
Functional outcomes were analyzed for patients who responded to the preoperative questionnaire; 331 of the 545 patients responded to the FISI and IPSS questionnaires preoperatively, and 73 responded to the IIEF-5 questionnaire preoperatively. The number of patients who responded at all 4 time points approximately 36 months postoperatively was 233, 269, and 10 for the FISI, IPSS, and IIEF-5 questionnaires, respectively. There were no differences in the pattern of change in scores over time for FISI score, IPSS, and IIEF-5 score between the 2 groups; however, when comparing the changes in scores preoperatively and 12 months postoperatively between the 2 groups, there was only a significant difference in the FISI score (0 [range, –31 to 45] vs. 0 [range, –16 to 45], P=0.023) (Table 5). We analyzed changes in defecatory and urogenital functions at each point (Fig. 3).
DISCUSSION
This study suggested no significant differences in oncological outcomes, including OS and RFS, between the HL and LL groups. In addition, there were no differences in postoperative complications, such as anastomotic leakage and recurrence patterns, between the 2 groups. Regarding functional outcomes, it was possible to confirm the pattern of change postoperatively; however, no significant difference was observed between the 2 groups. Notably, the LL group showed a more significant recovery of defecatory function than the HL group approximately 12 months postoperatively.
Furthermore, several studies have reported more harvested LNs in the HL group [22, 24]. The present study also showed significantly more harvested LNs in the HL group than in the LL group; however, no significant difference in the number of harvested LNs (≥12) was observed between the 2 groups. Considering the variables affecting survival revealed by univariate analysis, it was confirmed that ≥12 harvested LNs did not affect survival. Therefore, although there may be differences in the number of harvested LNs, depending on the IMA ligation level, this does not seem to affect the oncological outcome. The length of the PRM was longer in the HL group, and splenic flexure mobilization was performed more frequently in the HL group. This is because HL is more capable of complete mobilization of the proximal colonic limb than LL; therefore, it can be interpreted that sufficient PRM was secured by additionally performing splenic flexure mobilization while selectively performing HL in patients with short proximal colonic limbs. Conversely, the shorter DRM length in the HL group could be attributed to selection bias, in which surgeons tend to opt for HL in patients with large sized tumors or lower tumor locations.
Furthermore, several studies have compared the oncological outcomes of the 2 ligation methods for rectal cancer. Slanetz and Grimson [12] and Singh et al. [13] reported that HL had tumor stage-specific benefits over LL. According to a study by Slanetz and Grimson [12] in 1997, the 5-year survival rate was higher in the HL group according to Duke’s classification B and C of rectal cancer patients (Duke’s B: HL [83.9%] vs. LL [73.9%], P<0.01; Duke’s C: HL [52.9%] vs. LL [42.5%], P<0.05), and Singh et al. [13] reported in 2017 that HL had more significant benefits than LL in terms of OS in the IMA positive LNs group (HR, 0.77; 95% CI, 0.66–0.89). However, several recent studies have reported no difference in survival rate according to the ligation level when performing LL with LN dissection around the IMA root [15, 16, 24, 26–28]. As a theoretical concept, HL had a better oncological outcome than LL while dissecting the mesentery from the root of the IMA; however, LL, which preserved the left colic artery while dissecting the LN around the root of the IMA, did not have a worse oncological outcome than HL, which is consistent with the present study.
Anastomotic leakage is one of the most important complications after rectal cancer surgery. The incidence of anastomotic leakage after rectal cancer surgery is 6.0% to 17.0% [29]. According to the studies by Hinoi et al. [30] and Chen et al. [31], IMA ligation level can act as a risk factor for the occurrence of anastomotic leakage, and this may be associated with the reduction in blood flow supplied to the anastomotic site according to the preservation of the left colic artery. According to Seike et al. [8], who studied the change in blood flow according to the IMA ligation level using laser Doppler, the HL and LL groups demonstrated a decrease in blood flow of 38.5%±1.8% and 16.4%±1.8%, respectively. In particular, older male patients showed >50% reduction in blood flow. This result supports the role of blood supply in the anastomotic leakage rate. A randomized controlled trial was conducted in 2018 by Fujii et al. [20] to investigate the difference in the rate of anastomotic leakage according to IMA ligation. However, there was no significant difference in the anastomotic leakage rate according to the IMA ligation level. These results are consistent with those of several other recent retrospective studies. Consistent with previous studies, the present study found no difference in anastomotic leakage according to the IMA ligation level.
Furthermore, several randomized clinical trials have been conducted regarding functional outcomes. In 2015, Matsuda et al. [32] conducted a randomized clinical trial comparing defecatory function according to the IMA ligation level and reported no significant differences in the self-assessment of defecation, the Fecal Incontinence Quality of Life (FIQL) scale, and Wexner incontinence score. However, FIQL and Wexner scores improved at 1 year than at 3 months in the LL group. A recent retrospective study by Fiori et al. [33] reported that defecatory function was more preserved in the LL group than in the HL group and also showed that the FISI score improved significantly at 1 year compared with 3 months in the LL group than in the HL group. As shown in Fig. 3A, both groups showed deterioration in defecatory function as the FISI score increased until 3 months postoperatively; however, after 3 months, the FISI score gradually decreased, and the groups showed improvement in defecatory function. At 1 year postoperatively, the FISI score was lower in the LL group than in the HL group, with a statistically significant difference between the 2 groups (P<0.023). This indicates that the recovery of defecatory function was better in the LL group than in the HL group 1 year postoperatively. Furthermore, several studies have been conducted to determine whether LL is better for defecatory function than HL; however, it seems necessary to pay attention to the study conducted by Koda et al. [9] in 2005, in which the longer the proximal colonic limb was denervated in the HL group, the more often spastic waves occurred. These spastic waves were associated with defecatory dysfunction (urgency, multiple evacuations, and major soiling). This result supports that defecatory function recovery was better in the LL group than in the HL group. Regarding urinary and sexual functions, a randomized controlled trial by Mari et al. [34] reported that urinary and sexual functions were better preserved in the LL group. However, in the present study, there was no significant difference in urinary and sexual functions between the 2 IMA ligation levels. According to a report by Heald and Ryall [35] and Havenga et al. [36], damage to the inferior hypogastric plexus and nerve must be minimized to preserve urinary and sexual functions, which is important in performing an accurate TME. There may be a correlation with damage to the superior hypogastric plexus passing around the IMA root when LNs are dissected around the IMA root; however, Nano et al. [37] demonstrated the safety of ligation around the IMA root by showing the running position of the autonomic nerve passing around the IMA root using a cadaveric study. Therefore, preserving urinary and sexual functions seems important in performing an accurate TME rather than the IMA ligation level.
This study had some limitations. First, it was a retrospective study conducted at a single institution, which may have caused an inherent potential for bias. A selection bias caused differences in tumor size, length of PRM, DRM, and splenic flexure mobilization between the 2 groups; therefore, we used multivariate analysis to evaluate the factors affecting oncological outcomes. However, these variables did not affect oncological outcomes. Additionally, it should be noted that data for classifying complications using the Clavien-Dindo classification or evaluating defecatory function with the low anterior syndrome score were not obtainable and therefore could not be included in this study. Second, the only method used for evaluating urinary function in female patients in this study was via the IPSS questionnaire. While the International Consultation on Incontinence Questionnaire (ICIQ) is generally considered a more appropriate tool for assessing urinary function in female patients, in this study, both male and female patients were evaluated using the IPSS. Third, a small sample size was used for analyzing functional outcomes. In particular, only 73 of the 545 patients participated in the evaluation of sexual function, and only 10 responded to the questionnaire at 36 months. Therefore, the data were insufficient to detect differences in sexual function between the 2 groups. However, postoperative changes in defecatory and urogenital functions were observed over time, and significant changes in defecatory function were observed in the LL group.
In conclusion, our study suggested that LL with LN dissection around the root of the IMA might not affect the oncologic outcomes compared to HL; however, it probably possesses minimal benefits for functional outcomes such as defecatory function. Therefore, well-designed randomized controlled trials are warranted to provide definite evidence for the benefits of LL.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study was supported by a grant from the National Cancer Center of Korea (No. 2110201-3).
Author contributions
Conceptualization: JHO; Data curation: JHO, MWL; Formal analysis: MWL, DEL; Funding acquisition: SCP; Investigation: MWL; Methodology: JHO, MWL; Project administration: JHO, MWL; Resources: JHO, SCP, DWL, HJC, DYK; Software: KY, DEL; Supervision: JHO, SCP, DWL, SSP, KY, KSH, DKS, CWH, BK, BCK, HJC, DYK; Validation: JHO, MWL; Visualization: MWL, DEL; Writing–original draft: MWL; Writing–review & editing: JHO, SCP, DWL, SSP, KY, KSH, DKS, CWH, BK, BCK, HJC, DYK, DWL. All authors read and approved the final manuscript.