- Search
| Ann Coloproctol > Volume 40(1); 2024 > Article |
|
Abstract
Purpose
Methods
Results
Notes
Funding
This study was supported by a grant from the National Cancer Center of Korea (No. 2110201-3).
Author contributions
Conceptualization: JHO; Data curation: JHO, MWL; Formal analysis: MWL, DEL; Funding acquisition: SCP; Investigation: MWL; Methodology: JHO, MWL; Project administration: JHO, MWL; Resources: JHO, SCP, DWL, HJC, DYK; Software: KY, DEL; Supervision: JHO, SCP, DWL, SSP, KY, KSH, DKS, CWH, BK, BCK, HJC, DYK; Validation: JHO, MWL; Visualization: MWL, DEL; Writing–original draft: MWL; Writing–review & editing: JHO, SCP, DWL, SSP, KY, KSH, DKS, CWH, BK, BCK, HJC, DYK, DWL. All authors read and approved the final manuscript.
Fig. 2.

Fig. 3.
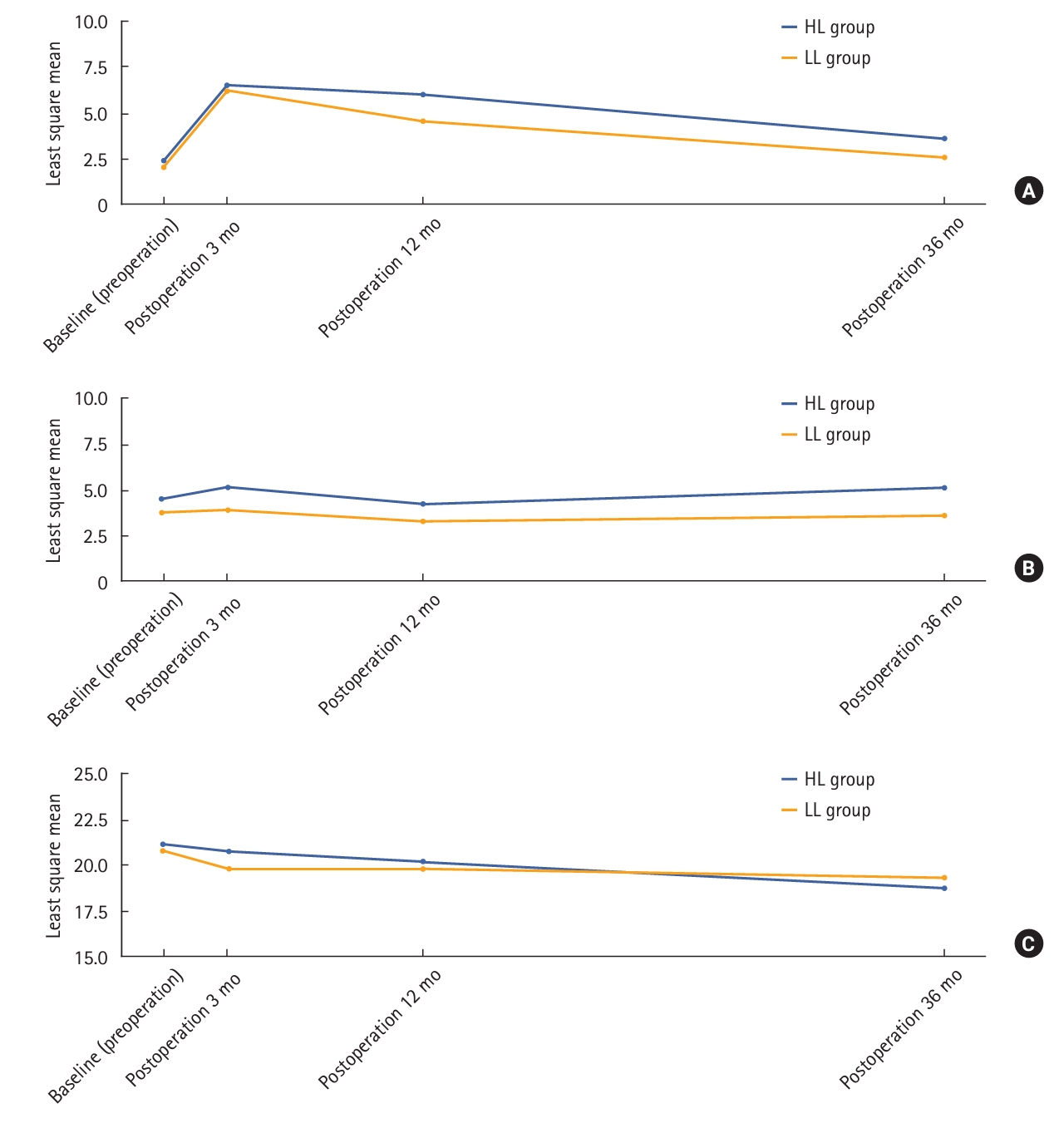
Table 1.
Values are presented as mean±standard deviation or number (%). P-values of continuous variables were calculated using 2 sample t-test or Wilcoxon rank sum test, whereas those of categorical variables were calculated using chi-square test or Fisher exact test.
HL, high ligation; LL, low ligation; RT, radiotherapy; AV, anal verge; LN, lymph node; PRM, proximal resection margin; DRM, distal resection margin.
Table 2.
Table 3.
| Variable | HL group (n=244) | LL group (n=301) | P-value* |
|---|---|---|---|
| Complication | 47 (19.3) | 57 (18.9) | >0.999 |
| Superficial or deep incisional SSI | 3 (1.2) | 2 (0.7) | 0.661 |
| Postoperative bleeding | 1 (0.4) | 5 (1.7) | 0.448 |
| Anastomosis site bleeding | 0 (0) | 3 (1.0) | |
| Intra-abdominal bleeding | 1 (0.4) | 2 (0.7) | |
| Ileus | 12 (4.9) | 20 (6.6) | 0.503 |
| Anastomotic leakage | 11 (4.5) | 13 (4.3) | >0.999 |
| Urinary retention | 9 (3.7) | 9 (3.0) | 0.832 |
| Ischemic colitis | 3 (1.3) | 1 (0.3) | 0.329 |
| Other | 0.863 | ||
| Acute pyelonephritis | 0 (0) | 1 (0.3) | |
| Cellulitis | 1 (0.4) | 0 (0) | |
| Cystitis | 1 (0.4) | 1 (0.3) | |
| Delirium | 1 (0.4) | 1 (0.3) | |
| Necrotizing fasciitis | 0 (0) | 1 (0.3) | |
| Perianal abscess | 0 (0) | 1 (0.3) | |
| Rectovaginal fistula | 0 (0) | 2 (0.7) |
Table 4.
| Variable | HL group (n=244) | LL group (n=301) | P-valuea* |
|---|---|---|---|
| Recurrence | 35 (14.3) | 48 (15.9) | 0.691 |
| Recurrence site | 0.195 | ||
| Local | 0 (0) | 4 (1.3) | |
| Systemic | 30 (12.3) | 29 (9.6) | |
| Local and systemic | 3 (1.2) | 7 (2.3) | |
| Local recurrence site | 0.334 | ||
| Anterior | 1 (0.4) | 1 (0.3) | |
| Posterior | 0 (0) | 3 (1.0) | |
| Lateral | 2 (0.8) | 6 (2.0) | |
| Anastomosis | 0 (0) | 1 (0.3) | |
| Systemic recurrence site | |||
| Liver | 10 (4.1) | 14 (4.7) | 0.918 |
| Lung | 24 (9.8) | 24 (8.0) | 0.541 |
| Peritoneal seeding | 4 (1.6) | 5 (1.7) | >0.999 |
| Bone | 2 (0.8) | 2 (0.7) | >0.999 |
| Para-aortic LNs | 5 (2.0) | 2 (0.7) | 0.252 |
Table 5.
| Difference |
HL group (n=244) |
LL group (n=301) |
P-value* | |||
|---|---|---|---|---|---|---|
| No. of patients | Median (range) | No. of patients | Median (range) | |||
| Fecal Incontinence Severity Index | ||||||
| Postoperation 3 mo − Preoperation | 124 | 0 (–31 to −49) | 135 | 0 (–16 to 49) | 0.359 | |
| Postoperation 12 mo − Preoperation | 120 | 0 (–31 to 45) | 167 | 0 (–16 to 45) | 0.023 | |
| Postoperation 36 mo − Preoperation | 91 | 0 (–31 to 32) | 124 | 0 (–13 to 20) | 0.071 | |
| International Prostate Symptom Score | ||||||
| Postoperation 3 mo − Preoperation | 129 | 0 (–22 to 17) | 174 | 0 (–14 to 25) | 0.296 | |
| Postoperation 12 mo − Preoperation | 120 | 0 (–22 to 29) | 166 | 0 (–26 to 26) | 0.602 | |
| Postoperation 36 mo − Preoperation | 93 | 0 (–20 to 23) | 124 | 0 (–14 to 25) | 0.368 | |
| International Index of Erectile Function | ||||||
| Postoperation 3 mo − Preoperation | 8 | –0.5 (–11 to 13) | 18 | 0 (–13 to 8) | 0.537 | |
| Postoperation 12 mo − Preoperation | 17 | 0 (–13 to 9) | 18 | 0 (–9 to 8) | 0.879 | |
| Postoperation 36 mo − Preoperation | 9 | 0 (–1 to 5) | 13 | 0 (–6 to 4) | 0.260 | |
REFERENCES
-
METRICS

-
- 0 Crossref
- 1 Scopus
- 2,057 View
- 179 Download
- Related articles in ACP